Interface Engineering of Earth-Abundant Transition Metals Using Boron Nitride for Selective Electroreduction of CO2
Abstract
Two-dimensional atomically thin hexagonal boron nitride (h-BN) monolayers have attracted considerable research interest. Given the tremendous progress in the synthesis of h-BN monolayers on transition metals and their potential as electrocatalysts, we investigate the electrocatalytic activities of h-BN/Ni, h-BN/Co, and h-BN/Cu interfaces for CO2 reduction by the first-principles density functional theory. We find that with the h-BN monolayer on the metal, electrons transfer from the metal to the interface and accumulate under the B atoms. By calculating the binding energies of three key intermediates (H, HCOO, and COOH) for hydrogen evolution and CO2 reduction, we find that H binding on the metal can be significantly weakened by the h-BN monolayer, preventing the hydrogen evolution reaction (HER). However, the binding strength of HCOO is strong on both the metal and h-BN/metal, especially for Ni and Co, promoting the CO2 reduction channel. On the basis of the free-energy diagrams, we predict that h-BN/Ni and h-BN/Co will have very good electrocatalytic activities for CO2 reduction to HCOOH, while the competitive HER channel is filtered out by the surface h-BN monolayer. Our study opens a new way for selective electroreduction of CO2 via the interface engineering of the h-BN/metal system.
- Authors:
-
- Univ. of California, Riverside, CA (United States). Dept. of Chemistry
- Oak Ridge National Lab. (ORNL), Oak Ridge, TN (United States). Chemical Sciences Division. Center for Nanophase Materials Sciences
- Oak Ridge National Lab. (ORNL), Oak Ridge, TN (United States). Chemical Sciences Division. Center for Nanophase Materials Sciences; Univ. of Tennessee, Knoxville, TN (United States). Dept. of Chemistry
- Publication Date:
- Research Org.:
- Oak Ridge National Laboratory (ORNL), Oak Ridge, TN (United States); Univ. of California, Riverside, CA (United States); Lawrence Berkeley National Laboratory (LBNL), Berkeley, CA (United States). National Energy Research Scientific Computing Center (NERSC)
- Sponsoring Org.:
- USDOE Office of Science (SC), Basic Energy Sciences (BES). Chemical Sciences, Geosciences, and Biosciences Division
- OSTI Identifier:
- 1468197
- Alternate Identifier(s):
- OSTI ID: 1485129
- Grant/Contract Number:
- AC05-00OR22725; AC02-05CH11231
- Resource Type:
- Accepted Manuscript
- Journal Name:
- ACS Applied Materials and Interfaces
- Additional Journal Information:
- Journal Volume: 10; Journal Issue: 7; Journal ID: ISSN 1944-8244
- Publisher:
- American Chemical Society (ACS)
- Country of Publication:
- United States
- Language:
- English
- Subject:
- 36 MATERIALS SCIENCE; earth-abundant transition metals; electrochemical CO2 reduction; first-principles DFT; h-BN monolayer; hydrogen evolution reaction; interfacial materials
Citation Formats
Hu, Guoxiang, Wu, Zili, Dai, Sheng, and Jiang, De-en. Interface Engineering of Earth-Abundant Transition Metals Using Boron Nitride for Selective Electroreduction of CO2. United States: N. p., 2018.
Web. doi:10.1021/acsami.7b17600.
Hu, Guoxiang, Wu, Zili, Dai, Sheng, & Jiang, De-en. Interface Engineering of Earth-Abundant Transition Metals Using Boron Nitride for Selective Electroreduction of CO2. United States. https://doi.org/10.1021/acsami.7b17600
Hu, Guoxiang, Wu, Zili, Dai, Sheng, and Jiang, De-en. Thu .
"Interface Engineering of Earth-Abundant Transition Metals Using Boron Nitride for Selective Electroreduction of CO2". United States. https://doi.org/10.1021/acsami.7b17600. https://www.osti.gov/servlets/purl/1468197.
@article{osti_1468197,
title = {Interface Engineering of Earth-Abundant Transition Metals Using Boron Nitride for Selective Electroreduction of CO2},
author = {Hu, Guoxiang and Wu, Zili and Dai, Sheng and Jiang, De-en},
abstractNote = {Two-dimensional atomically thin hexagonal boron nitride (h-BN) monolayers have attracted considerable research interest. Given the tremendous progress in the synthesis of h-BN monolayers on transition metals and their potential as electrocatalysts, we investigate the electrocatalytic activities of h-BN/Ni, h-BN/Co, and h-BN/Cu interfaces for CO2 reduction by the first-principles density functional theory. We find that with the h-BN monolayer on the metal, electrons transfer from the metal to the interface and accumulate under the B atoms. By calculating the binding energies of three key intermediates (H, HCOO, and COOH) for hydrogen evolution and CO2 reduction, we find that H binding on the metal can be significantly weakened by the h-BN monolayer, preventing the hydrogen evolution reaction (HER). However, the binding strength of HCOO is strong on both the metal and h-BN/metal, especially for Ni and Co, promoting the CO2 reduction channel. On the basis of the free-energy diagrams, we predict that h-BN/Ni and h-BN/Co will have very good electrocatalytic activities for CO2 reduction to HCOOH, while the competitive HER channel is filtered out by the surface h-BN monolayer. Our study opens a new way for selective electroreduction of CO2 via the interface engineering of the h-BN/metal system.},
doi = {10.1021/acsami.7b17600},
journal = {ACS Applied Materials and Interfaces},
number = 7,
volume = 10,
place = {United States},
year = {Thu Feb 01 00:00:00 EST 2018},
month = {Thu Feb 01 00:00:00 EST 2018}
}
Web of Science
Figures / Tables:
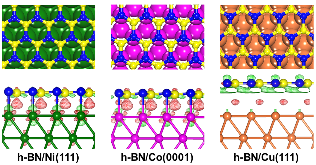 Figure 1: Top and side views of the optimized structures for h-BN/Ni, h-BN/Co, and h-BN/Cu. In the side view, the charge density difference plots are also shown. The red color indicates charge accumulation and the green color indicates charge depletion. The value of isosurface for h-BN/Ni(111) and h-BN/Co(0001) is 0.004more »
Figure 1: Top and side views of the optimized structures for h-BN/Ni, h-BN/Co, and h-BN/Cu. In the side view, the charge density difference plots are also shown. The red color indicates charge accumulation and the green color indicates charge depletion. The value of isosurface for h-BN/Ni(111) and h-BN/Co(0001) is 0.004more »
Works referenced in this record:
Hexagonal boron nitride: Fabrication, properties and applications
journal, January 1989
- Lipp, A.; Schwetz, K. A.; Hunold, K.
- Journal of the European Ceramic Society, Vol. 5, Issue 1
Direct-bandgap properties and evidence for ultraviolet lasing of hexagonal boron nitride single crystal
journal, May 2004
- Watanabe, Kenji; Taniguchi, Takashi; Kanda, Hisao
- Nature Materials, Vol. 3, Issue 6
Boron nitride finds new applications in thermoplastic compounds
journal, May 2008
- Raman, Chandrashekar; Meneghetti, Paulo
- Plastics, Additives and Compounding, Vol. 10, Issue 3
Far-ultraviolet plane-emission handheld device based on hexagonal boron nitride
journal, September 2009
- Watanabe, Kenji; Taniguchi, Takashi; Niiyama, Takahiro
- Nature Photonics, Vol. 3, Issue 10
Selective oxidative dehydrogenation of propane to propene using boron nitride catalysts
journal, December 2016
- Grant, J. T.; Carrero, C. A.; Goeltl, F.
- Science, Vol. 354, Issue 6319
Two-terminal floating-gate memory with van der Waals heterostructures for ultrahigh on/off ratio
journal, September 2016
- Vu, Quoc An; Shin, Yong Seon; Kim, Young Rae
- Nature Communications, Vol. 7, Issue 1
Atomistic Insights into Nucleation and Formation of Hexagonal Boron Nitride on Nickel from First-Principles-Based Reactive Molecular Dynamics Simulations
journal, March 2017
- Liu, Song; van Duin, Adri C. T.; van Duin, Diana M.
- ACS Nano, Vol. 11, Issue 4
Catalytic Directional Cutting of Hexagonal Boron Nitride: The Roles of Interface and Etching Agents
journal, April 2017
- Ma, Liang; Zeng, Xiao Cheng
- Nano Letters, Vol. 17, Issue 5
Irreparable Defects Produced by the Patching of h -BN Frontiers on Strongly Interacting Re(0001) and Their Electronic Properties
journal, April 2017
- Qi, Yue; Zhang, Zhepeng; Deng, Bing
- Journal of the American Chemical Society, Vol. 139, Issue 16
Atomic layers of hybridized boron nitride and graphene domains
journal, February 2010
- Ci, Lijie; Song, Li; Jin, Chuanhong
- Nature Materials, Vol. 9, Issue 5, p. 430-435
Boron nitride substrates for high-quality graphene electronics
journal, August 2010
- Dean, C. R.; Young, A. F.; Meric, I.
- Nature Nanotechnology, Vol. 5, Issue 10, p. 722-726
Electron Tunneling through Ultrathin Boron Nitride Crystalline Barriers
journal, February 2012
- Britnell, Liam; Gorbachev, Roman V.; Jalil, Rashid
- Nano Letters, Vol. 12, Issue 3
Visualizing Strain-Induced Pseudomagnetic Fields in Graphene through an hBN Magnifying Glass
journal, April 2017
- Jiang, Yuhang; Mao, Jinhai; Duan, Junxi
- Nano Letters, Vol. 17, Issue 5
Rocking-motion-induced charging of on
journal, March 2005
- Muntwiler, M.; Auwärter, W.; Seitsonen, A. P.
- Physical Review B, Vol. 71, Issue 12
The Quantum Magnetism of Individual Manganese-12-Acetate Molecular Magnets Anchored at Surfaces
journal, December 2011
- Kahle, Steffen; Deng, Zhitao; Malinowski, Nikola
- Nano Letters, Vol. 12, Issue 1
Taming interfacial electronic properties of platinum nanoparticles on vacancy-abundant boron nitride nanosheets for enhanced catalysis
journal, June 2017
- Zhu, Wenshuai; Wu, Zili; Foo, Guo Shiou
- Nature Communications, Vol. 8, Issue 1
Single Mo Atom Supported on Defective Boron Nitride Monolayer as an Efficient Electrocatalyst for Nitrogen Fixation: A Computational Study
journal, August 2017
- Zhao, Jingxiang; Chen, Zhongfang
- Journal of the American Chemical Society, Vol. 139, Issue 36
Synthesis of Few-Layer Hexagonal Boron Nitride Thin Film by Chemical Vapor Deposition
journal, October 2010
- Shi, Yumeng; Hamsen, Christoph; Jia, Xiaoting
- Nano Letters, Vol. 10, Issue 10
Large Scale Growth and Characterization of Atomic Hexagonal Boron Nitride Layers
journal, August 2010
- Song, Li; Ci, Lijie; Lu, Hao
- Nano Letters, Vol. 10, Issue 8, p. 3209-3215
Synthesis of Monolayer Hexagonal Boron Nitride on Cu Foil Using Chemical Vapor Deposition
journal, December 2011
- Kim, Ki Kang; Hsu, Allen; Jia, Xiaoting
- Nano Letters, Vol. 12, Issue 1
Chemical Vapor Deposition and Etching of High-Quality Monolayer Hexagonal Boron Nitride Films
journal, August 2011
- Sutter, Peter; Lahiri, Jayeeta; Albrecht, Peter
- ACS Nano, Vol. 5, Issue 9
Toward the Controlled Synthesis of Hexagonal Boron Nitride Films
journal, June 2012
- Ismach, Ariel; Chou, Harry; Ferrer, Domingo A.
- ACS Nano, Vol. 6, Issue 7
Formation of single layer h-BN on Pd(111)
journal, August 2006
- Morscher, M.; Corso, M.; Greber, T.
- Surface Science, Vol. 600, Issue 16
One-Dimensional Corrugation of the h -BN Monolayer on Fe(110)
journal, January 2012
- Vinogradov, N. A.; Zakharov, A. A.; Ng, M. L.
- Langmuir, Vol. 28, Issue 3
Boron Nitride Nanomesh: Functionality from a Corrugated Monolayer
journal, July 2007
- Berner, Simon; Corso, Martina; Widmer, Roland
- Angewandte Chemie International Edition, Vol. 46, Issue 27
Boron Nitride Nanosheet on Gold as an Electrocatalyst for Oxygen Reduction Reaction: Theoretical Suggestion and Experimental Proof
journal, April 2014
- Uosaki, Kohei; Elumalai, Ganesan; Noguchi, Hidenori
- Journal of the American Chemical Society, Vol. 136, Issue 18
Hexagonal Boron Nitride Cover on Pt(111): A New Route to Tune Molecule–Metal Interaction and Metal-Catalyzed Reactions
journal, April 2015
- Zhang, Yanhong; Weng, Xuefei; Li, Huan
- Nano Letters, Vol. 15, Issue 5
Catalysis with two-dimensional materials and their heterostructures
journal, March 2016
- Deng, Dehui; Novoselov, K. S.; Fu, Qiang
- Nature Nanotechnology, Vol. 11, Issue 3
When Inert Becomes Active: A Fascinating Route for Catalyst Design
journal, July 2016
- Lyalin, Andrey; Gao, Min; Taketsugu, Tetsuya
- The Chemical Record, Vol. 16, Issue 5
Hexagonal boron nitride on transition metal surfaces
journal, February 2013
- Gómez Díaz, Jaime; Ding, Yun; Koitz, Ralph
- Theoretical Chemistry Accounts, Vol. 132, Issue 4
PRODUCTION OF CO AND CH 4 IN ELECTROCHEMICAL REDUCTION OF CO 2 AT METAL ELECTRODES IN AQUEOUS HYDROGENCARBONATE SOLUTION
journal, November 1985
- Hori, Yoshio; Kikuchi, Katsuhei; Suzuki, Shin
- Chemistry Letters, Vol. 14, Issue 11
Electrochemical Reduction of Carbon Dioxide at Various Metal Electrodes in Aqueous Potassium Hydrogen Carbonate Solution
journal, September 1990
- Noda, Hidetomo; Ikeda, Shoichiro; Oda, Yoshiyuki
- Bulletin of the Chemical Society of Japan, Vol. 63, Issue 9, p. 2459-2462
Electrochemical CO2 Reduction on Metal Electrodes
book, January 2008
- Hori, Y.; Vayenas, Constantinos G.; White, Ralph E.
- Modern Aspects of Electrochemistry, p. 89-189
Electrochemical conversion of CO2 to useful chemicals: current status, remaining challenges, and future opportunities
journal, May 2013
- Jhong, Huei-Ru “Molly”; Ma, Sichao; Kenis, Paul JA
- Current Opinion in Chemical Engineering, Vol. 2, Issue 2
Activity Descriptors for CO 2 Electroreduction to Methane on Transition-Metal Catalysts
journal, January 2012
- Peterson, Andrew A.; Nørskov, Jens K.
- The Journal of Physical Chemistry Letters, Vol. 3, Issue 2
How copper catalyzes the electroreduction of carbon dioxide into hydrocarbon fuels
journal, January 2010
- Peterson, Andrew A.; Abild-Pedersen, Frank; Studt, Felix
- Energy & Environmental Science, Vol. 3, Issue 9
Trends in the Exchange Current for Hydrogen Evolution
journal, January 2005
- Nørskov, J. K.; Bligaard, T.; Logadottir, A.
- Journal of The Electrochemical Society, Vol. 152, Issue 3
First-Principles Analysis of the Initial Electroreduction Steps of Oxygen over Pt(111)
journal, January 2009
- Janik, Michael J.; Taylor, Christopher D.; Neurock, Matthew
- Journal of The Electrochemical Society, Vol. 156, Issue 1
The oxygen reduction reaction mechanism on Pt(111) from density functional theory calculations
journal, November 2010
- Tripković, Vladimir; Skúlason, Egill; Siahrostami, Samira
- Electrochimica Acta, Vol. 55, Issue 27
Highly Efficient Electrochemical Hydrogen Evolution Reaction at Insulating Boron Nitride Nanosheet on Inert Gold Substrate
journal, August 2016
- Uosaki, Kohei; Elumalai, Ganesan; Dinh, Hung Cuong
- Scientific Reports, Vol. 6, Issue 1
A grid-based Bader analysis algorithm without lattice bias
journal, January 2009
- Tang, W.; Sanville, E.; Henkelman, G.
- Journal of Physics: Condensed Matter, Vol. 21, Issue 8
A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu
journal, April 2010
- Grimme, Stefan; Antony, Jens; Ehrlich, Stephan
- The Journal of Chemical Physics, Vol. 132, Issue 15
Works referencing / citing this record:
Mo-doped boron nitride monolayer as a promising single-atom electrocatalyst for CO 2 conversion
journal, January 2019
- Cui, Qianyi; Qin, Gangqiang; Wang, Weihua
- Beilstein Journal of Nanotechnology, Vol. 10

 Search WorldCat to find libraries that may hold this journal
Search WorldCat to find libraries that may hold this journal