Surface Ligand Promotion of Carbon Dioxide Reduction through Stabilizing Chemisorbed Reactive Intermediates
Abstract
We have explored functionalizing metal catalysts with surface ligands as an approach to facilitate electrochemical carbon dioxide reduction reaction (CO2RR). To provide a molecular level understanding of the mechanism by which this enhancement occurs, we combine in situ spectroscopy analysis with an interpretation based on quantum mechanics (QM) calculations. We find that a surface ligand can play a critical role in stabilizing the chemisorbed CO2, which facilitates CO2 activation and leads to a 0.3 V decrease in the overpotential for carbon monoxide (CO) formation. Moreover, the presence of the surface ligand leads to nearly exclusive CO production. At -0.6 V (versus reversible hydrogen electrode, RHE), CO is the only significant product with a faradic efficiency of 93% and a current density of 1.9 mA cm–2. This improvement corresponds to 53-fold enhancement in turnover frequency compared with the Ag nanoparticles (NPs) without surface ligands.
- Authors:
-
- Harbin Inst. of Technology (China)
- Harbin Medical Univ. (China)
- Univ. of Oxford (United Kingdom)
- California Inst. of Technology (CalTech), Pasadena, CA (United States)
- Publication Date:
- Research Org.:
- California Institute of Technology (CalTech), Pasadena, CA (United States)
- Sponsoring Org.:
- USDOE Office of Science (SC)
- OSTI Identifier:
- 1467603
- Grant/Contract Number:
- SC0004993
- Resource Type:
- Accepted Manuscript
- Journal Name:
- Journal of Physical Chemistry Letters
- Additional Journal Information:
- Journal Volume: 9; Journal Issue: 11; Journal ID: ISSN 1948-7185
- Publisher:
- American Chemical Society
- Country of Publication:
- United States
- Language:
- English
- Subject:
- 37 INORGANIC, ORGANIC, PHYSICAL, AND ANALYTICAL CHEMISTRY
Citation Formats
Wang, Zhijiang, Wu, Lina, Sun, Kun, Chen, Ting, Jiang, Zhaohua, Cheng, Tao, and Goddard, III, William A. Surface Ligand Promotion of Carbon Dioxide Reduction through Stabilizing Chemisorbed Reactive Intermediates. United States: N. p., 2018.
Web. doi:10.1021/acs.jpclett.8b00959.
Wang, Zhijiang, Wu, Lina, Sun, Kun, Chen, Ting, Jiang, Zhaohua, Cheng, Tao, & Goddard, III, William A. Surface Ligand Promotion of Carbon Dioxide Reduction through Stabilizing Chemisorbed Reactive Intermediates. United States. https://doi.org/10.1021/acs.jpclett.8b00959
Wang, Zhijiang, Wu, Lina, Sun, Kun, Chen, Ting, Jiang, Zhaohua, Cheng, Tao, and Goddard, III, William A. Tue .
"Surface Ligand Promotion of Carbon Dioxide Reduction through Stabilizing Chemisorbed Reactive Intermediates". United States. https://doi.org/10.1021/acs.jpclett.8b00959. https://www.osti.gov/servlets/purl/1467603.
@article{osti_1467603,
title = {Surface Ligand Promotion of Carbon Dioxide Reduction through Stabilizing Chemisorbed Reactive Intermediates},
author = {Wang, Zhijiang and Wu, Lina and Sun, Kun and Chen, Ting and Jiang, Zhaohua and Cheng, Tao and Goddard, III, William A.},
abstractNote = {We have explored functionalizing metal catalysts with surface ligands as an approach to facilitate electrochemical carbon dioxide reduction reaction (CO2RR). To provide a molecular level understanding of the mechanism by which this enhancement occurs, we combine in situ spectroscopy analysis with an interpretation based on quantum mechanics (QM) calculations. We find that a surface ligand can play a critical role in stabilizing the chemisorbed CO2, which facilitates CO2 activation and leads to a 0.3 V decrease in the overpotential for carbon monoxide (CO) formation. Moreover, the presence of the surface ligand leads to nearly exclusive CO production. At -0.6 V (versus reversible hydrogen electrode, RHE), CO is the only significant product with a faradic efficiency of 93% and a current density of 1.9 mA cm–2. This improvement corresponds to 53-fold enhancement in turnover frequency compared with the Ag nanoparticles (NPs) without surface ligands.},
doi = {10.1021/acs.jpclett.8b00959},
journal = {Journal of Physical Chemistry Letters},
number = 11,
volume = 9,
place = {United States},
year = {Tue May 22 00:00:00 EDT 2018},
month = {Tue May 22 00:00:00 EDT 2018}
}
Web of Science
Figures / Tables:
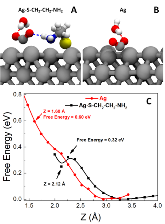 Figure 1: (A) chemisorbed CO2 on cysteamine functionalized Ag(111) surface. (B) Chemisorbed CO2 on Ag(111) surface. (C) The free energy profile of the CO2 approaching surface. Only the water molecules directly forming hydrogen bond are shown (the remaining 31 or 30 solvent water molecules are removed for clarity). The hydrogenmore »
Figure 1: (A) chemisorbed CO2 on cysteamine functionalized Ag(111) surface. (B) Chemisorbed CO2 on Ag(111) surface. (C) The free energy profile of the CO2 approaching surface. Only the water molecules directly forming hydrogen bond are shown (the remaining 31 or 30 solvent water molecules are removed for clarity). The hydrogenmore »
Works referenced in this record:
Anthropogenic Chemical Carbon Cycle for a Sustainable Future
journal, August 2011
- Olah, George A.; Prakash, G. K. Surya; Goeppert, Alain
- Journal of the American Chemical Society, Vol. 133, Issue 33
Observational determination of surface radiative forcing by CO2 from 2000 to 2010
journal, February 2015
- Feldman, D. R.; Collins, W. D.; Gero, P. J.
- Nature, Vol. 519, Issue 7543
Ionic Liquid-Mediated Selective Conversion of CO2 to CO at Low Overpotentials
journal, September 2011
- Rosen, B. A.; Salehi-Khojin, A.; Thorson, M. R.
- Science, Vol. 334, Issue 6056, p. 643-644
Electrocatalytic and homogeneous approaches to conversion of CO 2 to liquid fuels
journal, January 2009
- Benson, Eric E.; Kubiak, Clifford P.; Sathrum, Aaron J.
- Chem. Soc. Rev., Vol. 38, Issue 1
Status and perspectives of CO2 conversion into fuels and chemicals by catalytic, photocatalytic and electrocatalytic processes
journal, January 2013
- Kondratenko, Evgenii V.; Mul, Guido; Baltrusaitis, Jonas
- Energy & Environmental Science, Vol. 6, Issue 11
Electrocatalytic Conversion of Carbon Dioxide to Methane and Methanol on Transition Metal Surfaces
journal, August 2014
- Kuhl, Kendra P.; Hatsukade, Toru; Cave, Etosha R.
- Journal of the American Chemical Society, Vol. 136, Issue 40
Unravelling charge carrier dynamics in protonated g-C3N4 interfaced with carbon nanodots as co-catalysts toward enhanced photocatalytic CO2 reduction: A combined experimental and first-principles DFT study
journal, February 2017
- Ong, Wee-Jun; Putri, Lutfi Kurnianditia; Tan, Yoong-Chuen
- Nano Research, Vol. 10, Issue 5
Ti 2 CO 2 MXene: a highly active and selective photocatalyst for CO 2 reduction
journal, January 2017
- Zhang, Xu; Zhang, Zihe; Li, Jielan
- Journal of Materials Chemistry A, Vol. 5, Issue 25
Understanding of Electrochemical Mechanisms for CO 2 Capture and Conversion into Hydrocarbon Fuels in Transition-Metal Carbides (MXenes)
journal, September 2017
- Li, Neng; Chen, Xingzhu; Ong, Wee-Jun
- ACS Nano, Vol. 11, Issue 11
A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels
journal, January 2014
- Qiao, Jinli; Liu, Yuyu; Hong, Feng
- Chem. Soc. Rev., Vol. 43, Issue 2
Enhanced electrocatalytic CO2 reduction via field-induced reagent concentration
journal, August 2016
- Liu, Min; Pang, Yuanjie; Zhang, Bo
- Nature, Vol. 537, Issue 7620
Synergistic geometric and electronic effects for electrochemical reduction of carbon dioxide using gold–copper bimetallic nanoparticles
journal, September 2014
- Kim, Dohyung; Resasco, Joaquin; Yu, Yi
- Nature Communications, Vol. 5, Issue 1
A selective and efficient electrocatalyst for carbon dioxide reduction
journal, January 2014
- Lu, Qi; Rosen, Jonathan; Zhou, Yang
- Nature Communications, Vol. 5, Issue 1
Monodisperse Au Nanoparticles for Selective Electrocatalytic Reduction of CO 2 to CO
journal, October 2013
- Zhu, Wenlei; Michalsky, Ronald; Metin, Önder
- Journal of the American Chemical Society, Vol. 135, Issue 45
Achieving Selective and Efficient Electrocatalytic Activity for CO 2 Reduction Using Immobilized Silver Nanoparticles
journal, October 2015
- Kim, Cheonghee; Jeon, Hyo Sang; Eom, Taedaehyeong
- Journal of the American Chemical Society, Vol. 137, Issue 43
Electrochemical Reduction of CO 2 at Functionalized Au Electrodes
journal, February 2017
- Fang, Yuxin; Flake, John C.
- Journal of the American Chemical Society, Vol. 139, Issue 9
Nitrogen-Based Catalysts for the Electrochemical Reduction of CO 2 to CO
journal, November 2012
- Tornow, Claire E.; Thorson, Michael R.; Ma, Sichao
- Journal of the American Chemical Society, Vol. 134, Issue 48
In Situ Spectroscopic Examination of a Low Overpotential Pathway for Carbon Dioxide Conversion to Carbon Monoxide
journal, July 2012
- Rosen, Brian A.; Haan, John L.; Mukherjee, Prabuddha
- The Journal of Physical Chemistry C, Vol. 116, Issue 29, p. 15307-15312
Electrochemical CO2 reduction: Electrocatalyst, reaction mechanism, and process engineering
journal, November 2016
- Lu, Qi; Jiao, Feng
- Nano Energy, Vol. 29
Ultrahigh Mass Activity for Carbon Dioxide Reduction Enabled by Gold–Iron Core–Shell Nanoparticles
journal, October 2017
- Sun, Kun; Cheng, Tao; Wu, Lina
- Journal of the American Chemical Society, Vol. 139, Issue 44
Shape-Dependent Electrocatalytic Reduction of CO 2 to CO on Triangular Silver Nanoplates
journal, February 2017
- Liu, Subiao; Tao, Hongbiao; Zeng, Li
- Journal of the American Chemical Society, Vol. 139, Issue 6
Structure, Function, and Mechanism of the Nickel Metalloenzymes, CO Dehydrogenase, and Acetyl-CoA Synthase
journal, February 2014
- Can, Mehmet; Armstrong, Fraser A.; Ragsdale, Stephen W.
- Chemical Reviews, Vol. 114, Issue 8
Understanding Trends in the Electrocatalytic Activity of Metals and Enzymes for CO 2 Reduction to CO
journal, January 2013
- Hansen, Heine A.; Varley, Joel B.; Peterson, Andrew A.
- The Journal of Physical Chemistry Letters, Vol. 4, Issue 3
Reaction Mechanisms for the Electrochemical Reduction of CO 2 to CO and Formate on the Cu(100) Surface at 298 K from Quantum Mechanics Free Energy Calculations with Explicit Water
journal, October 2016
- Cheng, Tao; Xiao, Hai; Goddard, William A.
- Journal of the American Chemical Society, Vol. 138, Issue 42
Nature of the Active Sites for CO Reduction on Copper Nanoparticles; Suggestions for Optimizing Performance
journal, August 2017
- Cheng, Tao; Xiao, Hai; Goddard, William A.
- Journal of the American Chemical Society, Vol. 139, Issue 34
Influence of electrolytes on the structure of cysteamine monolayer on silver studied by surface-enhanced Raman scattering
journal, January 2001
- Michota, Agnieszka; Kudelski, Andrzej; Bukowska, Jolanta
- Journal of Raman Spectroscopy, Vol. 32, Issue 5
Structures of monolayers formed from different HS?(CH2)2?X thiols on gold, silver and copper: comparitive studies by surface-enhanced Raman scattering
journal, January 2003
- Kudelski, Andrzej
- Journal of Raman Spectroscopy, Vol. 34, Issue 11
Raman Study on the Structure of Cysteamine Monolayers on Silver
journal, April 1999
- Kudelski, Andrzej; Hill, Wieland
- Langmuir, Vol. 15, Issue 9
In Situ Surface-Enhanced Raman Spectroscopy of the Electrochemical Reduction of Carbon Dioxide on Silver with 3,5-Diamino-1,2,4-Triazole
journal, July 2014
- Schmitt, Kevin G.; Gewirth, Andrew A.
- The Journal of Physical Chemistry C, Vol. 118, Issue 31
Raman cross section of some simple gases
journal, January 1973
- Fenner, Wayne R.; Hyatt, Howard A.; Kellam, John M.
- Journal of the Optical Society of America, Vol. 63, Issue 1
Matrix isolation and ab initio study on HCN/CO 2 system and its radiation-induced transformations: Spectroscopic evidence for HCN⋯CO 2 and trans -HCNH⋯CO 2 complexes
journal, December 2016
- Kameneva, Svetlana V.; Tyurin, Daniil A.; Nuzhdin, Kirill B.
- The Journal of Chemical Physics, Vol. 145, Issue 21
Effect of Chloride Anions on the Synthesis and Enhanced Catalytic Activity of Silver Nanocoral Electrodes for CO 2 Electroreduction
journal, August 2015
- Hsieh, Yu-Chi; Senanayake, Sanjaya D.; Zhang, Yu
- ACS Catalysis, Vol. 5, Issue 9
Works referencing / citing this record:
Polydopamine Functionalized Cu Nanowires for Enhanced CO 2 Electroreduction Towards Methane
journal, October 2018
- Liu, Hui; Xiang, Kaisong; Liu, Yucheng
- ChemElectroChem, Vol. 5, Issue 24
Understanding the role of functional groups of thiolate ligands in electrochemical CO 2 reduction over Au(111) from first-principles
journal, January 2019
- Li, Fuhua; Tang, Qing
- Journal of Materials Chemistry A, Vol. 7, Issue 34
Tuning Gold Nanoparticles with Chelating Ligands for Highly Efficient Electrocatalytic CO 2 Reduction
journal, August 2018
- Cao, Zhi; Zacate, Samson B.; Sun, Xiaodong
- Angewandte Chemie, Vol. 130, Issue 39
Tuning Structural and Compositional Effects in Pd-Au Nanowires for Highly Selective and Active CO 2 Electrochemical Reduction Reaction
journal, October 2018
- Zhu, Shangqian; Wang, Qi; Qin, Xueping
- Advanced Energy Materials, Vol. 8, Issue 32
Electronic structure benchmark calculations of inorganic and biochemical carboxylation reactions
journal, February 2019
- Douglas‐Gallardo, Oscar A.; Saez, David Adrian; Vogt‐Geisse, Stefan
- Journal of Computational Chemistry, Vol. 40, Issue 13
Biosynthesized silver nanorings as a highly efficient and selective electrocatalysts for CO 2 reduction
journal, January 2019
- Pan, Yani; Paschoalino, Waldemir J.; Bayram, Serene S.
- Nanoscale, Vol. 11, Issue 40
Tuning Gold Nanoparticles with Chelating Ligands for Highly Efficient Electrocatalytic CO 2 Reduction
journal, August 2018
- Cao, Zhi; Zacate, Samson B.; Sun, Xiaodong
- Angewandte Chemie International Edition, Vol. 57, Issue 39
Figures / Tables found in this record:

 Search WorldCat to find libraries that may hold this journal
Search WorldCat to find libraries that may hold this journal