Molecular modeling and assignment of IR spectra of the hydrated excess proton in isotopically dilute water
Abstract
Infrared (IR) spectroscopy of the water O–H stretch has been widely used to probe both the local hydrogen-bonding structure and dynamics of aqueous systems. Although of significant interest, the IR spectroscopy of excess protons in water remains difficult to assign as a result of extensive and strong intermolecular interactions in hydrated proton complexes. As an alternate approach, we develop a mixed quantum-classical model for the vibrational spectroscopy of the excess proton in isotopically dilute water that draws on frozen proton-water clusters taken from reactive molecular dynamics trajectories of the latest generation multi-state empirical valence bond proton model (MS-EVB 3.2). A semi-empirical single oscillator spectroscopic map for the instantaneous transition frequency and transition dipole moment is constructed using potential energy surfaces for the O–H stretch coordinate of the excess proton using electronic structure calculations. Calculated spectra are compared with experimental spectra of dilute H+ in D2O obtained from double-difference FTIR to demonstrate the validity of the map. The model is also used to decompose IR spectra into contributions from different aqueous proton configurations. We find that the O–H transition frequency continuously decreases as the oxygen-oxygen length for a special pair proton decreases, shifting from Eigen- to Zundel-like configurations. The same shiftmore »
- Authors:
-
- Univ. of Chicago, IL (United States). Dept. of Chemistry, James Franck Inst., and Inst. for Biophysical Dynamics
- Publication Date:
- Research Org.:
- Univ. of Chicago, IL (United States). Dept. Chemistry, James Franck Institute, and Institute for Biophysical Dynamics
- Sponsoring Org.:
- USDOE Office of Science (SC), Basic Energy Sciences (BES). Chemical Sciences, Geosciences, and Biosciences Division; National Science Foundation (NSF)
- OSTI Identifier:
- 1465106
- Alternate Identifier(s):
- OSTI ID: 1329330; OSTI ID: 1582240
- Grant/Contract Number:
- SC0005418; ACI-1053575; SC0014305
- Resource Type:
- Accepted Manuscript
- Journal Name:
- Journal of Chemical Physics
- Additional Journal Information:
- Journal Volume: 145; Journal Issue: 15; Journal ID: ISSN 0021-9606
- Publisher:
- American Institute of Physics (AIP)
- Country of Publication:
- United States
- Language:
- English
- Subject:
- 37 INORGANIC, ORGANIC, PHYSICAL, AND ANALYTICAL CHEMISTRY; 71 CLASSICAL AND QUANTUM MECHANICS, GENERAL PHYSICS
Citation Formats
Biswas, Rajib, Carpenter, William, Voth, Gregory A., and Tokmakoff, Andrei. Molecular modeling and assignment of IR spectra of the hydrated excess proton in isotopically dilute water. United States: N. p., 2016.
Web. doi:10.1063/1.4964723.
Biswas, Rajib, Carpenter, William, Voth, Gregory A., & Tokmakoff, Andrei. Molecular modeling and assignment of IR spectra of the hydrated excess proton in isotopically dilute water. United States. https://doi.org/10.1063/1.4964723
Biswas, Rajib, Carpenter, William, Voth, Gregory A., and Tokmakoff, Andrei. Wed .
"Molecular modeling and assignment of IR spectra of the hydrated excess proton in isotopically dilute water". United States. https://doi.org/10.1063/1.4964723. https://www.osti.gov/servlets/purl/1465106.
@article{osti_1465106,
title = {Molecular modeling and assignment of IR spectra of the hydrated excess proton in isotopically dilute water},
author = {Biswas, Rajib and Carpenter, William and Voth, Gregory A. and Tokmakoff, Andrei},
abstractNote = {Infrared (IR) spectroscopy of the water O–H stretch has been widely used to probe both the local hydrogen-bonding structure and dynamics of aqueous systems. Although of significant interest, the IR spectroscopy of excess protons in water remains difficult to assign as a result of extensive and strong intermolecular interactions in hydrated proton complexes. As an alternate approach, we develop a mixed quantum-classical model for the vibrational spectroscopy of the excess proton in isotopically dilute water that draws on frozen proton-water clusters taken from reactive molecular dynamics trajectories of the latest generation multi-state empirical valence bond proton model (MS-EVB 3.2). A semi-empirical single oscillator spectroscopic map for the instantaneous transition frequency and transition dipole moment is constructed using potential energy surfaces for the O–H stretch coordinate of the excess proton using electronic structure calculations. Calculated spectra are compared with experimental spectra of dilute H+ in D2O obtained from double-difference FTIR to demonstrate the validity of the map. The model is also used to decompose IR spectra into contributions from different aqueous proton configurations. We find that the O–H transition frequency continuously decreases as the oxygen-oxygen length for a special pair proton decreases, shifting from Eigen- to Zundel-like configurations. The same shift is accompanied by a shift of the flanking water stretches of the Zundel complex to higher frequency than the hydronium O–H vibrations.},
doi = {10.1063/1.4964723},
journal = {Journal of Chemical Physics},
number = 15,
volume = 145,
place = {United States},
year = {Wed Oct 19 00:00:00 EDT 2016},
month = {Wed Oct 19 00:00:00 EDT 2016}
}
Web of Science
Figures / Tables:
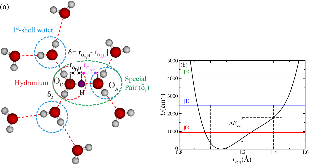 FIG. 1: (a) Illustration of solvated hydronium ion (red circle) showing the proton donor (D) and acceptor (A) molecules of a tagged O–H · · ·O bond. The proton sharing parameter $δ$ is calculated for each tagged proton (purple). The smallest $δ$ value determines the special pair (green ellipse) andmore »
FIG. 1: (a) Illustration of solvated hydronium ion (red circle) showing the proton donor (D) and acceptor (A) molecules of a tagged O–H · · ·O bond. The proton sharing parameter $δ$ is calculated for each tagged proton (purple). The smallest $δ$ value determines the special pair (green ellipse) andmore »
Works referenced in this record:
Observation of a Zundel-like transition state during proton transfer in aqueous hydroxide solutions
journal, July 2009
- Roberts, S. T.; Petersen, P. B.; Ramasesha, K.
- Proceedings of the National Academy of Sciences, Vol. 106, Issue 36
Transport in Proton Conductors for Fuel-Cell Applications: Simulations, Elementary Reactions, and Phenomenology
journal, October 2004
- Kreuer, Klaus-Dieter; Paddison, Stephen J.; Spohr, Eckhard
- Chemical Reviews, Vol. 104, Issue 10
IR and Raman spectra of liquid water: Theory and interpretation
journal, June 2008
- Auer, B. M.; Skinner, J. L.
- The Journal of Chemical Physics, Vol. 128, Issue 22
A THz/FTIR fingerprint of the solvated proton: evidence for Eigen structure and Zundel dynamics
journal, January 2015
- Decka, Dominique; Schwaab, Gerhard; Havenith, Martina
- Physical Chemistry Chemical Physics, Vol. 17, Issue 17
Molecular Mechanism of HCl Acid Ionization in Water: Ab Initio Potential Energy Surfaces and Monte Carlo Simulations
journal, December 1997
- Ando, Koji; Hynes, James T.
- The Journal of Physical Chemistry B, Vol. 101, Issue 49
Theoretical simulation of OH and OD stretching bands of isotopically diluted HDO molecules in aqueous solution
journal, April 1993
- Wojcik, Marek J.; Hermansson, Kersti; Lindgren, Jan
- Chemical Physics, Vol. 171, Issue 1-2
Calculated frequencies and intensities associated with coupling of the proton motion with the hydrogen bond stretching vibration in a double minimum potential surface
journal, January 1973
- Janoschek, R.; Weidemann, Erich G.; Zundel, Georg
- Journal of the Chemical Society, Faraday Transactions 2, Vol. 69
Time-resolved observation of the Eigen cation in liquid water
journal, January 2007
- Amir, Wafa; Gallot, Guilhem; Hache, François
- The Journal of Chemical Physics, Vol. 126, Issue 3
Electric Field Fluctuations Drive Vibrational Dephasing in Water
journal, October 2005
- Eaves, Joel D.; Tokmakoff, Andrei; Geissler, Phillip L.
- The Journal of Physical Chemistry A, Vol. 109, Issue 42
Both Zundel and Eigen Isomers Contribute to the IR Spectrum of the Gas-Phase H 9 O 4 + Cluster
journal, December 2013
- Kulig, Waldemar; Agmon, Noam
- The Journal of Physical Chemistry B, Vol. 118, Issue 1
Role of Charge Transfer in the Structure and Dynamics of the Hydrated Proton
journal, April 2009
- Swanson, Jessica M. J.; Simons, Jack
- The Journal of Physical Chemistry B, Vol. 113, Issue 15
The Structure of the Hydrogen Ion (H aq + ) in Water
journal, February 2010
- Stoyanov, Evgenii S.; Stoyanova, Irina V.; Reed, Christopher A.
- Journal of the American Chemical Society, Vol. 132, Issue 5
Deciphering the infrared spectrum of the protonated water pentamer and the hybrid Eigen–Zundel cation
journal, January 2014
- Kulig, Waldemar; Agmon, Noam
- Physical Chemistry Chemical Physics, Vol. 16, Issue 10
Ultrafast 2D IR spectroscopy of the excess proton in liquid water
journal, October 2015
- Thämer, Martin; De Marco, Luigi; Ramasesha, Krupa
- Science, Vol. 350, Issue 6256
An Improved Multistate Empirical Valence Bond Model for Aqueous Proton Solvation and Transport †
journal, January 2008
- Wu, Yujie; Chen, Hanning; Wang, Feng
- The Journal of Physical Chemistry B, Vol. 112, Issue 2
Unraveling Anharmonic Effects in the Vibrational Predissociation Spectra of H 5 O 2 + and Its Deuterated Analogues
journal, June 2011
- Guasco, Timothy L.; Johnson, Mark A.; McCoy, Anne B.
- The Journal of Physical Chemistry A, Vol. 115, Issue 23
Water vibrations have strongly mixed intra- and intermolecular character
journal, September 2013
- Ramasesha, Krupa; De Marco, Luigi; Mandal, Aritra
- Nature Chemistry, Vol. 5, Issue 11
The nature of the hydrated excess proton in water
journal, February 1999
- Marx, Dominik; Tuckerman, Mark E.; Hutter, Jürg
- Nature, Vol. 397, Issue 6720
Hydrogen Bonds with Large Proton Polarizability and Proton Transfer Processes in Electrochemistry and Biology
book, January 1999
- Zundel, Georg
- Advances in Chemical Physics
An analysis of hydrated proton diffusion in ab initio molecular dynamics
journal, January 2015
- Tse, Ying-Lung Steve; Knight, Chris; Voth, Gregory A.
- The Journal of Chemical Physics, Vol. 142, Issue 1
Proton Conduction in Exchange Membranes across Multiple Length Scales
journal, March 2012
- Jorn, Ryan; Savage, John; Voth, Gregory A.
- Accounts of Chemical Research, Vol. 45, Issue 11
The Molecular Origin of the “Continuous” Infrared Absorption in Aqueous Solutions of Acids: A Computational Approach
journal, February 2006
- Iftimie, Radu; Tuckerman, Mark E.
- Angewandte Chemie International Edition, Vol. 45, Issue 7
Et tu, Grotthuss! and other unfinished stories
journal, August 2006
- Cukierman, Samuel
- Biochimica et Biophysica Acta (BBA) - Bioenergetics, Vol. 1757, Issue 8
Effect of Environment on Hydrogen Bond Dynamics in Liquid Water
journal, February 1996
- Luzar, Alenka; Chandler, David
- Physical Review Letters, Vol. 76, Issue 6
Ab initio molecular-dynamics simulation of aqueous proton solvation and transport revisited
journal, July 2005
- Izvekov, Sergei; Voth, Gregory A.
- The Journal of Chemical Physics, Vol. 123, Issue 4
Experimental Evidence of Fermi Resonances in Isotopically Dilute Water from Ultrafast Broadband IR Spectroscopy
journal, May 2013
- De Marco, Luigi; Ramasesha, Krupa; Tokmakoff, Andrei
- The Journal of Physical Chemistry B, Vol. 117, Issue 49
A statistical mechanical theory of proton transport kinetics in hydrogen-bonded networks based on population correlation functions with applications to acids and bases
journal, September 2010
- Tuckerman, Mark E.; Chandra, Amalendu; Marx, Dominik
- The Journal of Chemical Physics, Vol. 133, Issue 12
Flexible simple point-charge water model with improved liquid-state properties
journal, January 2006
- Wu, Yujie; Tepper, Harald L.; Voth, Gregory A.
- The Journal of Chemical Physics, Vol. 124, Issue 2
A bond-order analysis of the mechanism for hydrated proton mobility in liquid water
journal, January 2005
- Lapid, Hadas; Agmon, Noam; Petersen, Matt K.
- The Journal of Chemical Physics, Vol. 122, Issue 1
Connecting Solvation Shell Structure to Proton Transport Kinetics in Hydrogen–Bonded Networks via Population Correlation Functions
journal, October 2007
- Chandra, Amalendu; Tuckerman, Mark E.; Marx, Dominik
- Physical Review Letters, Vol. 99, Issue 14
Spectral Signatures of Hydrated Proton Vibrations in Water Clusters
journal, June 2005
- Headrick, J. M.
- Science, Vol. 308, Issue 5729
Special Pair Dance and Partner Selection: Elementary Steps in Proton Transport in Liquid Water
journal, August 2008
- Markovitch, Omer; Chen, Hanning; Izvekov, Sergei
- The Journal of Physical Chemistry B, Vol. 112, Issue 31
Role of Presolvation and Anharmonicity in Aqueous Phase Hydrated Proton Solvation and Transport
journal, November 2015
- Biswas, Rajib; Tse, Ying-Lung Steve; Tokmakoff, Andrei
- The Journal of Physical Chemistry B, Vol. 120, Issue 8
H+ and OH− ions in aqueous solutions vibrational spectra of hydrates
journal, June 1979
- Librovich, N. B.; Sakun, V. P.; Sokolov, N. D.
- Chemical Physics, Vol. 39, Issue 3
The Curious Case of the Hydrated Proton
journal, August 2011
- Knight, Chris; Voth, Gregory A.
- Accounts of Chemical Research, Vol. 45, Issue 1
The vibrational spectrum of the hydrated proton: Comparison of experiment, simulation, and normal mode analysis
journal, January 2002
- Kim, Jeongho; Schmitt, Udo W.; Gruetzmacher, Julie A.
- The Journal of Chemical Physics, Vol. 116, Issue 2
Gas-Phase Infrared Spectrum of the Protonated Water Dimer
journal, February 2003
- Asmis, K. R.
- Science, Vol. 299, Issue 5611
Infrared Spectra of HCl(H 2 O) n Clusters from Semiempirical Born–Oppenheimer Molecular Dynamics Simulations
journal, November 2014
- Lin, Wei; Paesani, Francesco
- The Journal of Physical Chemistry A, Vol. 119, Issue 19
Concerted Hydrogen-Bond Dynamics in the Transport Mechanism of the Hydrated Proton: A First-Principles Molecular Dynamics Study
journal, November 2009
- Berkelbach, Timothy C.; Lee, Hee-Seung; Tuckerman, Mark E.
- Physical Review Letters, Vol. 103, Issue 23
Proton Transfer, Acid-Base Catalysis, and Enzymatic Hydrolysis. Part I: ELEMENTARY PROCESSES
journal, January 1964
- Eigen, M.
- Angewandte Chemie International Edition in English, Vol. 3, Issue 1
A molecular dynamics method for simulations in the canonical ensemble
journal, June 1984
- Nosé, Shūichi
- Molecular Physics, Vol. 52, Issue 2
Infrared and Raman Line Shapes of Dilute HOD in Liquid H 2 O and D 2 O from 10 to 90 °C
journal, July 2005
- Corcelli, S. A.; Skinner, J. L.
- The Journal of Physical Chemistry A, Vol. 109, Issue 28
Proton transfer through the water gossamer
journal, July 2013
- Hassanali, Ali; Giberti, Federico; Cuny, Jérôme
- Proceedings of the National Academy of Sciences, Vol. 110, Issue 34
Proton Transfer 200 Years after von Grotthuss: Insights from Ab Initio Simulations
journal, September 2006
- Marx, Dominik
- ChemPhysChem, Vol. 7, Issue 9
Ultrafast Vibrational and Structural Dynamics of the Proton in Liquid Water
journal, April 2006
- Woutersen, Sander; Bakker, Huib J.
- Physical Review Letters, Vol. 96, Issue 13
Proton Solvation and Transport in Aqueous and Biomolecular Systems: Insights from Computer Simulations
journal, May 2007
- Swanson, Jessica M. J.; Maupin, C. Mark; Chen, Hanning
- The Journal of Physical Chemistry B, Vol. 111, Issue 17
Proton Transport Mechanism of Perfluorosulfonic Acid Membranes
journal, July 2014
- Savage, John; Tse, Ying-Lung Steve; Voth, Gregory A.
- The Journal of Physical Chemistry C, Vol. 118, Issue 31
Ab initio molecular dynamics simulation of the solvation and transport of hydronium and hydroxyl ions in water
journal, July 1995
- Tuckerman, M.; Laasonen, K.; Sprik, M.
- The Journal of Chemical Physics, Vol. 103, Issue 1
Fundamental Excitations of the Shared Proton in the H 3 O 2 - and H 5 O 2 + Complexes
journal, March 2005
- Diken, Eric G.; Headrick, Jeffrey M.; Roscioli, Joseph R.
- The Journal of Physical Chemistry A, Vol. 109, Issue 8
Hydrogen Bond Dynamics in Water and Ultrafast Infrared Spectroscopy
journal, December 2002
- Rey, Rossend; Møller, Klaus B.; Hynes, James T.
- The Journal of Physical Chemistry A, Vol. 106, Issue 50
Calculation of the Vibrational Spectra of H 5 O 2 + and Its Deuterium-Substituted Isotopologues by Molecular Dynamics Simulations †
journal, July 2009
- Kaledin, Martina; Kaledin, Alexey L.; Bowman, Joel M.
- The Journal of Physical Chemistry A, Vol. 113, Issue 26
Proton Transfer in Concentrated Aqueous Hydroxide Visualized Using Ultrafast Infrared Spectroscopy
journal, April 2011
- Roberts, Sean T.; Ramasesha, Krupa; Petersen, Poul B.
- The Journal of Physical Chemistry A, Vol. 115, Issue 16
Full dimensional (15-dimensional) quantum-dynamical simulation of the protonated water dimer. II. Infrared spectrum and vibrational dynamics
journal, November 2007
- Vendrell, Oriol; Gatti, Fabien; Meyer, Hans-Dieter
- The Journal of Chemical Physics, Vol. 127, Issue 18
The Grotthuss mechanism
journal, October 1995
- Agmon, Noam
- Chemical Physics Letters, Vol. 244, Issue 5-6
Transport and spectroscopy of the hydrated proton: A molecular dynamics study
journal, September 1999
- Vuilleumier, Rodolphe; Borgis, Daniel
- The Journal of Chemical Physics, Vol. 111, Issue 9
Mechanism and Thermodynamics of Ion Selectivity in Aqueous Solutions of 18-Crown-6 Ether: A Molecular Dynamics Study
journal, July 1995
- Dang, Liem X.
- Journal of the American Chemical Society, Vol. 117, Issue 26
Vibrational spectral signature of the proton defect in the three-dimensional H+(H2O)21 cluster
journal, May 2014
- Fournier, J. A.; Johnson, C. J.; Wolke, C. T.
- Science, Vol. 344, Issue 6187
The OH vibrational spectrum of liquid water from combined a b i n i t i o and Monte Carlo calculations
journal, November 1991
- Hermansson, Kersti; Knuts, Sören; Lindgren, Jan
- The Journal of Chemical Physics, Vol. 95, Issue 10
A novel discrete variable representation for quantum mechanical reactive scattering via the S ‐matrix Kohn method
journal, February 1992
- Colbert, Daniel T.; Miller, William H.
- The Journal of Chemical Physics, Vol. 96, Issue 3
Collective vibrations of water-solvated hydroxide ions investigated with broadband 2DIR spectroscopy
journal, May 2014
- Mandal, Aritra; Ramasesha, Krupa; De Marco, Luigi
- The Journal of Chemical Physics, Vol. 140, Issue 20
The computer simulation of proton transport in water
journal, November 1999
- Schmitt, Udo W.; Voth, Gregory A.
- The Journal of Chemical Physics, Vol. 111, Issue 20
An H/D Isotopic Substitution Study of the H 5 O 2 + ·Ar Vibrational Predissociation Spectra: Exploring the Putative Role of Fermi Resonances in the Bridging Proton Fundamentals †
journal, January 2008
- McCunn, Laura R.; Roscioli, Joseph R.; Johnson, Mark A.
- The Journal of Physical Chemistry B, Vol. 112, Issue 2
Robustness of Frequency, Transition Dipole, and Coupling Maps for Water Vibrational Spectroscopy
journal, June 2013
- Gruenbaum, S. M.; Tainter, C. J.; Shi, L.
- Journal of Chemical Theory and Computation, Vol. 9, Issue 7
Infrared Spectrum of the Hydrated Proton in Water
journal, December 2010
- Xu, Jianqing; Zhang, Yong; Voth, Gregory A.
- The Journal of Physical Chemistry Letters, Vol. 2, Issue 2
Infrared Spectra of a Model Phenol-Amine Proton Transfer Complex in Nanoconfined CH 3 Cl
journal, June 2008
- Mitchell-Koch, Katie R.; Thompson*, Ward H.
- The Journal of Physical Chemistry B, Vol. 112, Issue 25
Pronounced non-Condon effects in the ultrafast infrared spectroscopy of water
journal, July 2005
- Schmidt, J. R.; Corcelli, S. A.; Skinner, J. L.
- The Journal of Chemical Physics, Vol. 123, Issue 4
Computer Simulation of Proton Solvation and Transport in Aqueous and Biomolecular Systems
journal, February 2006
- Voth, Gregory A.
- Accounts of Chemical Research, Vol. 39, Issue 2
A ‘clusters-in-liquid’ method for calculating infrared spectra identifies the proton-transfer mode in acidic aqueous solutions
journal, November 2012
- Kulig, Waldemar; Agmon, Noam
- Nature Chemistry, Vol. 5, Issue 1
Works referencing / citing this record:
Recent advances in quantum‐mechanical molecular dynamics simulations of proton transfer mechanism in various water‐based environments
journal, May 2019
- Sakti, Aditya W.; Nishimura, Yoshifumi; Nakai, Hiromi
- WIREs Computational Molecular Science, Vol. 10, Issue 1
Broadband 2D IR spectroscopy reveals dominant asymmetric H5O2+ proton hydration structures in acid solutions
journal, July 2018
- Fournier, Joseph A.; Carpenter, William B.; Lewis, Nicholas H. C.
- Nature Chemistry, Vol. 10, Issue 9
Decoding the spectroscopic features and time scales of aqueous proton defects
journal, June 2018
- Napoli, Joseph A.; Marsalek, Ondrej; Markland, Thomas E.
- The Journal of Chemical Physics, Vol. 148, Issue 22
Figures / Tables found in this record:

 Search WorldCat to find libraries that may hold this journal
Search WorldCat to find libraries that may hold this journal