Mechanism of CO2 Reduction at Copper Surfaces: Pathways to C2 Products
Abstract
On the basis of constraints from reported experimental observations and density functional theory simulations, in this paper we propose a mechanism for the reduction of CO2 to C2 products on copper electrodes. To model the effects of an applied potential bias on the reactions, calculations are carried out with a variable, fractional number of electrons on the unit cell, which is optimized so that the Fermi level matches the actual chemical potential of electrons (i.e., the applied bias); an implicit electrolyte model allows for compensation of the surface charge so that neutrality is maintained in the overall simulation cell. Our mechanism explains the presence of the seven C2 species that have been detected in the reaction, as well as other notable experimental observations. Furthermore, our results shed light on the difference in activities toward C2 products between the (100) and (111) facets of copper. Finally, we compare our methodologies and findings with those in other recent mechanistic studies of the copper-catalyzed CO2 reduction reaction.
- Authors:
-
- Lawrence Berkeley National Lab. (LBNL), Berkeley, CA (United States). Joint Center for Artificial Photosynthesis
- Univ. of California, Berkeley, CA (United States). Dept. of Chemical and Biomolecular Engineering
- Univ. of California, Berkeley, CA (United States). Dept. of Chemistry
- Publication Date:
- Research Org.:
- Lawrence Berkeley National Laboratory (LBNL), Berkeley, CA (United States)
- Sponsoring Org.:
- USDOE Office of Science (SC), Basic Energy Sciences (BES)
- OSTI Identifier:
- 1464161
- Grant/Contract Number:
- AC02-05CH11231; SC0004993
- Resource Type:
- Accepted Manuscript
- Journal Name:
- ACS Catalysis
- Additional Journal Information:
- Journal Volume: 8; Journal Issue: 2; Journal ID: ISSN 2155-5435
- Publisher:
- American Chemical Society (ACS)
- Country of Publication:
- United States
- Language:
- English
- Subject:
- 37 INORGANIC, ORGANIC, PHYSICAL, AND ANALYTICAL CHEMISTRY; artificial photosynthesis; DFT; electrocatalysis; ethanol; ethylene; mechanisms
Citation Formats
Garza, Alejandro J., Bell, Alexis T., and Head-Gordon, Martin. Mechanism of CO2 Reduction at Copper Surfaces: Pathways to C2 Products. United States: N. p., 2018.
Web. doi:10.1021/acscatal.7b03477.
Garza, Alejandro J., Bell, Alexis T., & Head-Gordon, Martin. Mechanism of CO2 Reduction at Copper Surfaces: Pathways to C2 Products. United States. https://doi.org/10.1021/acscatal.7b03477
Garza, Alejandro J., Bell, Alexis T., and Head-Gordon, Martin. Thu .
"Mechanism of CO2 Reduction at Copper Surfaces: Pathways to C2 Products". United States. https://doi.org/10.1021/acscatal.7b03477. https://www.osti.gov/servlets/purl/1464161.
@article{osti_1464161,
title = {Mechanism of CO2 Reduction at Copper Surfaces: Pathways to C2 Products},
author = {Garza, Alejandro J. and Bell, Alexis T. and Head-Gordon, Martin},
abstractNote = {On the basis of constraints from reported experimental observations and density functional theory simulations, in this paper we propose a mechanism for the reduction of CO2 to C2 products on copper electrodes. To model the effects of an applied potential bias on the reactions, calculations are carried out with a variable, fractional number of electrons on the unit cell, which is optimized so that the Fermi level matches the actual chemical potential of electrons (i.e., the applied bias); an implicit electrolyte model allows for compensation of the surface charge so that neutrality is maintained in the overall simulation cell. Our mechanism explains the presence of the seven C2 species that have been detected in the reaction, as well as other notable experimental observations. Furthermore, our results shed light on the difference in activities toward C2 products between the (100) and (111) facets of copper. Finally, we compare our methodologies and findings with those in other recent mechanistic studies of the copper-catalyzed CO2 reduction reaction.},
doi = {10.1021/acscatal.7b03477},
journal = {ACS Catalysis},
number = 2,
volume = 8,
place = {United States},
year = {Thu Jan 11 00:00:00 EST 2018},
month = {Thu Jan 11 00:00:00 EST 2018}
}
Web of Science
Figures / Tables:
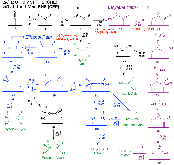 Figure 1:: Proposed mechanism for the reduction of CO to C2 products at high potentials on Cu(100). Calculated free energies (eV) are the numbers parallel to reaction arrows, where ∆G values at U = 0 V using the CHE appear in standard font (steps involving H+ + e− can bemore »
Figure 1:: Proposed mechanism for the reduction of CO to C2 products at high potentials on Cu(100). Calculated free energies (eV) are the numbers parallel to reaction arrows, where ∆G values at U = 0 V using the CHE appear in standard font (steps involving H+ + e− can bemore »
Works referenced in this record:
Production of Methane and Ethylene in Electrochemical Reduction of Carbon Dioxide at Copper Electrode in Aqueous Hydrogencarbonate Solution
journal, June 1986
- Hori, Yoshio; Kikuchi, Katsuhei; Murata, Akira
- Chemistry Letters, Vol. 15, Issue 6
Formation of hydrocarbons in the electrochemical reduction of carbon dioxide at a copper electrode in aqueous solution
journal, January 1989
- Hori, Yoshio; Murata, Akira; Takahashi, Ryutaro
- Journal of the Chemical Society, Faraday Transactions 1: Physical Chemistry in Condensed Phases, Vol. 85, Issue 8
New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces
journal, January 2012
- Kuhl, Kendra P.; Cave, Etosha R.; Abram, David N.
- Energy & Environmental Science, Vol. 5, Issue 5
Adsorption of CO accompanied with simultaneous charge transfer on copper single crystal electrodes related with electrochemical reduction of CO2 to hydrocarbons
journal, July 1995
- Hori, Y.; Wakebe, H.; Tsukamoto, T.
- Surface Science, Vol. 335
Electrochemical reduction of CO2 at copper single crystal Cu(S)-[n(111)×(111)] and Cu(S)-[n(110)×(100)] electrodes
journal, September 2002
- Takahashi, Ichiro; Koga, Osamu; Hoshi, Nagahiro
- Journal of Electroanalytical Chemistry, Vol. 533, Issue 1-2, p. 135-143
Electrochemical reduction of carbon dioxide at various series of copper single crystal electrodes
journal, May 2003
- Hori, Y.; Takahashi, I.; Koga, O.
- Journal of Molecular Catalysis A: Chemical, Vol. 199, Issue 1-2, p. 39-47
Prospects of CO2 Utilization via Direct Heterogeneous Electrochemical Reduction
journal, December 2010
- Whipple, Devin T.; Kenis, Paul J. A.
- The Journal of Physical Chemistry Letters, Vol. 1, Issue 24, p. 3451-3458
How copper catalyzes the electroreduction of carbon dioxide into hydrocarbon fuels
journal, January 2010
- Peterson, Andrew A.; Abild-Pedersen, Frank; Studt, Felix
- Energy & Environmental Science, Vol. 3, Issue 9
Selectivity of CO 2 Reduction on Copper Electrodes: The Role of the Kinetics of Elementary Steps
journal, January 2013
- Nie, Xiaowa; Esopi, Monica R.; Janik, Michael J.
- Angewandte Chemie International Edition, Vol. 52, Issue 9
Catalysts and Reaction Pathways for the Electrochemical Reduction of Carbon Dioxide
journal, September 2015
- Kortlever, Ruud; Shen, Jing; Schouten, Klaas Jan P.
- The Journal of Physical Chemistry Letters, Vol. 6, Issue 20
Mechanistic Insights into the Reduction of CO 2 on Tin Electrodes using in Situ ATR-IR Spectroscopy
journal, March 2015
- Baruch, Maor F.; Pander, James E.; White, James L.
- ACS Catalysis, Vol. 5, Issue 5
In Situ Spectroscopic Study of CO 2 Electroreduction at Copper Electrodes in Acetonitrile
journal, March 2016
- Figueiredo, Marta C.; Ledezma-Yanez, Isis; Koper, Marc T. M.
- ACS Catalysis, Vol. 6, Issue 4
A new mechanism for the selectivity to C1 and C2 species in the electrochemical reduction of carbon dioxide on copper electrodes
journal, January 2011
- Schouten, K. J. P.; Kwon, Y.; van der Ham, C. J. M.
- Chemical Science, Vol. 2, Issue 10
Free-Energy Barriers and Reaction Mechanisms for the Electrochemical Reduction of CO on the Cu(100) Surface, Including Multiple Layers of Explicit Solvent at pH 0
journal, November 2015
- Cheng, Tao; Xiao, Hai; Goddard, William A.
- The Journal of Physical Chemistry Letters, Vol. 6, Issue 23
Reaction Mechanisms for the Electrochemical Reduction of CO 2 to CO and Formate on the Cu(100) Surface at 298 K from Quantum Mechanics Free Energy Calculations with Explicit Water
journal, October 2016
- Cheng, Tao; Xiao, Hai; Goddard, William A.
- Journal of the American Chemical Society, Vol. 138, Issue 42
Facet Dependence of CO 2 Reduction Paths on Cu Electrodes
journal, December 2015
- Luo, Wenjia; Nie, Xiaowa; Janik, Michael J.
- ACS Catalysis, Vol. 6, Issue 1
Full atomistic reaction mechanism with kinetics for CO reduction on Cu(100) from ab initio molecular dynamics free-energy calculations at 298 K
journal, February 2017
- Cheng, Tao; Xiao, Hai; Goddard, William A.
- Proceedings of the National Academy of Sciences, Vol. 114, Issue 8
Theoretical Considerations on the Electroreduction of CO to C 2 Species on Cu(100) Electrodes
journal, June 2013
- Calle-Vallejo, Federico; Koper, Marc T. M.
- Angewandte Chemie International Edition, Vol. 52, Issue 28
Insights into CC Coupling in CO 2 Electroreduction on Copper Electrodes
journal, January 2013
- Montoya, Joseph H.; Peterson, Andrew A.; Nørskov, Jens K.
- ChemCatChem, Vol. 5, Issue 3
Theoretical Insights into a CO Dimerization Mechanism in CO 2 Electroreduction
journal, May 2015
- Montoya, Joseph H.; Shi, Chuan; Chan, Karen
- The Journal of Physical Chemistry Letters, Vol. 6, Issue 11
Identification of Possible Pathways for C–C Bond Formation during Electrochemical Reduction of CO 2 : New Theoretical Insights from an Improved Electrochemical Model
journal, April 2016
- Goodpaster, Jason D.; Bell, Alexis T.; Head-Gordon, Martin
- The Journal of Physical Chemistry Letters, Vol. 7, Issue 8
Structure-sensitive electroreduction of acetaldehyde to ethanol on copper and its mechanistic implications for CO and CO 2 reduction
journal, March 2016
- Ledezma-Yanez, Isis; Gallent, Elena Pérez; Koper, Marc T. M.
- Catalysis Today, Vol. 262
Subsurface oxide plays a critical role in CO 2 activation by Cu(111) surfaces to form chemisorbed CO 2 , the first step in reduction of CO 2
journal, June 2017
- Favaro, Marco; Xiao, Hai; Cheng, Tao
- Proceedings of the National Academy of Sciences
Cu metal embedded in oxidized matrix catalyst to promote CO 2 activation and CO dimerization for electrochemical reduction of CO 2
journal, June 2017
- Xiao, Hai; Goddard, William A.; Cheng, Tao
- Proceedings of the National Academy of Sciences
Enhanced Carbon Dioxide Electroreduction to Carbon Monoxide over Defect-Rich Plasma-Activated Silver Catalysts
journal, August 2017
- Mistry, Hemma; Choi, Yong-Wook; Bagger, Alexander
- Angewandte Chemie, Vol. 129, Issue 38
Electronic structure calculations of liquid-solid interfaces: Combination of density functional theory and modified Poisson-Boltzmann theory
journal, June 2008
- Jinnouchi, Ryosuke; Anderson, Alfred B.
- Physical Review B, Vol. 77, Issue 24
Electroreduction of carbon monoxide to liquid fuel on oxide-derived nanocrystalline copper
journal, April 2014
- Li, Christina W.; Ciston, Jim; Kanan, Matthew W.
- Nature, Vol. 508, Issue 7497
CO 2 Electroreduction with Enhanced Ethylene and Ethanol Selectivity by Nanostructuring Polycrystalline Copper
journal, April 2016
- Kwon, Youngkook; Lum, Yanwei; Clark, Ezra L.
- ChemElectroChem, Vol. 3, Issue 6
High-Selectivity Electrochemical Conversion of CO 2 to Ethanol using a Copper Nanoparticle/N-Doped Graphene Electrode
journal, September 2016
- Song, Yang; Peng, Rui; Hensley, Dale K.
- ChemistrySelect, Vol. 1, Issue 19
Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set
journal, October 1996
- Kresse, G.; Furthmüller, J.
- Physical Review B, Vol. 54, Issue 16, p. 11169-11186
A climbing image nudged elastic band method for finding saddle points and minimum energy paths
journal, December 2000
- Henkelman, Graeme; Uberuaga, Blas P.; Jónsson, Hannes
- The Journal of Chemical Physics, Vol. 113, Issue 22, p. 9901-9904
A generalized solid-state nudged elastic band method
journal, February 2012
- Sheppard, Daniel; Xiao, Penghao; Chemelewski, William
- The Journal of Chemical Physics, Vol. 136, Issue 7
A dimer method for finding saddle points on high dimensional potential surfaces using only first derivatives
journal, October 1999
- Henkelman, Graeme; Jónsson, Hannes
- The Journal of Chemical Physics, Vol. 111, Issue 15
Implicit solvation model for density-functional study of nanocrystal surfaces and reaction pathways
journal, February 2014
- Mathew, Kiran; Sundararaman, Ravishankar; Letchworth-Weaver, Kendra
- The Journal of Chemical Physics, Vol. 140, Issue 8
Joint density functional theory of the electrode-electrolyte interface: Application to fixed electrode potentials, interfacial capacitances, and potentials of zero charge
journal, August 2012
- Letchworth-Weaver, Kendra; Arias, T. A.
- Physical Review B, Vol. 86, Issue 7
Theoretical Study of Trans-metalation Process in Palladium-Catalyzed Borylation of Iodobenzene with Diboron
journal, August 2004
- Sumimoto, Michinori; Iwane, Naoki; Takahama, Tomohiko
- Journal of the American Chemical Society, Vol. 126, Issue 33
Mechanistic and Computational Study of a Palladacycle-Catalyzed Decomposition of a Series of Neutral Phosphorothioate Triesters in Methanol
journal, November 2010
- Liu, C. Tony; Maxwell, Christopher I.; Edwards, David R.
- Journal of the American Chemical Society, Vol. 132, Issue 46
Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode
journal, November 2004
- Nørskov, J. K.; Rossmeisl, J.; Logadottir, A.
- The Journal of Physical Chemistry B, Vol. 108, Issue 46
A simple method to approximate electrode potential-dependent activation energies using density functional theory
journal, June 2017
- Akhade, Sneha A.; Bernstein, Nicole J.; Esopi, Monica R.
- Catalysis Today, Vol. 288
Steric Effects in Electrolytes: A Modified Poisson-Boltzmann Equation
journal, July 1997
- Borukhov, Itamar; Andelman, David; Orland, Henri
- Physical Review Letters, Vol. 79, Issue 3
Adsorption of large ions from an electrolyte solution: a modified Poisson–Boltzmann equation
journal, November 2000
- Borukhov, I.; Andelman, D.; Orland, H.
- Electrochimica Acta, Vol. 46, Issue 2-3
Aqueous and Surface Redox Potentials from Self-Consistently Determined Gibbs Energies
journal, May 2008
- Jinnouchi, Ryosuke; Anderson, Alfred B.
- The Journal of Physical Chemistry C, Vol. 112, Issue 24
Reappraisal of thermochemical radii for complex ions
journal, September 1979
- Jenkins, H. D. B.; Thakur, K. P.
- Journal of Chemical Education, Vol. 56, Issue 9
Multigrid-Based Methodology for Implicit Solvation Models in Periodic DFT
journal, February 2016
- Garcia-Ratés, Miquel; López, Núria
- Journal of Chemical Theory and Computation, Vol. 12, Issue 3
First-Principles Determination of the Absolute Hydration Free Energy of the Hydroxide Ion †
journal, October 2002
- Zhan, Chang-Guo; Dixon, David A.
- The Journal of Physical Chemistry A, Vol. 106, Issue 42
Electroreduction of carbon monoxide to methane and ethylene at a copper electrode in aqueous solutions at ambient temperature and pressure
journal, August 1987
- Hori, Yoshio; Murata, Akira; Takahashi, Ryutaro
- Journal of the American Chemical Society, Vol. 109, Issue 16
Electrochemical Reduction of CO at a Copper Electrode
journal, September 1997
- Hori, Yoshio; Takahashi, Ryutaro; Yoshinami, Yuzuru
- The Journal of Physical Chemistry B, Vol. 101, Issue 36
A review of the aqueous electrochemical reduction of CO2 to hydrocarbons at copper
journal, August 2006
- Gattrell, M.; Gupta, N.; Co, A.
- Journal of Electroanalytical Chemistry, Vol. 594, Issue 1, p. 1-19
Reaction mechanisms of CO2 electrochemical reduction on Cu(111) determined with density functional theory
journal, April 2014
- Nie, Xiaowa; Luo, Wenjia; Janik, Michael J.
- Journal of Catalysis, Vol. 312
Structural Influences on Adsorption Energy. III. CO on Cu(100)
journal, March 1972
- Tracy, J. C.
- The Journal of Chemical Physics, Vol. 56, Issue 6
The Impact of Specifically Adsorbed Ions on the Copper-Catalyzed Electroreduction of CO 2
journal, January 2016
- Akhade, Sneha A.; McCrum, Ian T.; Janik, Michael J.
- Journal of The Electrochemical Society, Vol. 163, Issue 6
Theory of multiple proton–electron transfer reactions and its implications for electrocatalysis
journal, January 2013
- Koper, Marc T. M.
- Chemical Science, Vol. 4, Issue 7
Two Pathways for the Formation of Ethylene in CO Reduction on Single-Crystal Copper Electrodes
journal, June 2012
- Schouten, Klaas Jan P.; Qin, Zisheng; Pérez Gallent, Elena
- Journal of the American Chemical Society, Vol. 134, Issue 24
The electrochemical characterization of copper single-crystal electrodes in alkaline media
journal, June 2013
- P. Schouten, Klaas Jan; Gallent, Elena Pérez; Koper, Marc T. M.
- Journal of Electroanalytical Chemistry, Vol. 699
Structure Sensitivity of the Electrochemical Reduction of Carbon Monoxide on Copper Single Crystals
journal, April 2013
- Schouten, Klaas Jan P.; Pérez Gallent, Elena; Koper, Marc T. M.
- ACS Catalysis, Vol. 3, Issue 6
Electrochemical Barriers Made Simple
journal, June 2015
- Chan, Karen; Nørskov, Jens K.
- The Journal of Physical Chemistry Letters, Vol. 6, Issue 14
New reduction mechanism of CO dimer by hydrogenation to C 2 H 4 on a Cu(100) surface: theoretical insight into the kinetics of the elementary steps
journal, January 2015
- Ou, Lihui; Long, Wenqi; Chen, Yuandao
- RSC Advances, Vol. 5, Issue 117
Bond-Making and Breaking between Carbon, Nitrogen, and Oxygen in Electrocatalysis
journal, October 2014
- Li, Hongjiao; Li, Yongdan; Koper, Marc T. M.
- Journal of the American Chemical Society, Vol. 136, Issue 44
Structure and composition of Cu(hkl) surfaces exposed to O2 and emersed from alkaline solutions: Prelude to UHV-EC studies of CO2 reduction at well-defined copper catalysts
journal, March 2014
- Baricuatro, Jack H.; Ehlers, Charles B.; Cummins, Kyle D.
- Journal of Electroanalytical Chemistry, Vol. 716
Cathodic regeneration of a clean and ordered Cu(1 0 0)-(1×1) surface from an air-oxidized and disordered electrode: An operando STM study
journal, November 2014
- Kim, Youn-Geun; Soriaga, Manuel P.
- Journal of Electroanalytical Chemistry, Vol. 734
Regulating the Product Distribution of CO Reduction by the Atomic-Level Structural Modification of the Cu Electrode Surface
journal, June 2016
- Kim, Youn-Geun; Javier, Alnald; Baricuatro, Jack H.
- Electrocatalysis, Vol. 7, Issue 5
Revised Pourbaix Diagrams for Copper at 25 to 300°C
journal, January 1997
- Beverskog, B.
- Journal of The Electrochemical Society, Vol. 144, Issue 10
CO adsorption on Cu(111) and Cu(001) surfaces: Improving site preference in DFT calculations
journal, October 2005
- Gajdoš, Marek; Hafner, Jürgen
- Surface Science, Vol. 590, Issue 2-3
Spectroscopic Observation of a Hydrogenated CO Dimer Intermediate During CO Reduction on Cu(100) Electrodes
journal, February 2017
- Pérez-Gallent, Elena; Figueiredo, Marta C.; Calle-Vallejo, Federico
- Angewandte Chemie, Vol. 129, Issue 13
Structure- and Potential-Dependent Cation Effects on CO Reduction at Copper Single-Crystal Electrodes
journal, November 2017
- Pérez-Gallent, Elena; Marcandalli, Giulia; Figueiredo, Marta Costa
- Journal of the American Chemical Society, Vol. 139, Issue 45
Promoter Effects of Alkali Metal Cations on the Electrochemical Reduction of Carbon Dioxide
journal, August 2017
- Resasco, Joaquin; Chen, Leanne D.; Clark, Ezra
- Journal of the American Chemical Society, Vol. 139, Issue 32
The influence of pH on the reduction of CO and to hydrocarbons on copper electrodes
journal, March 2014
- Schouten, Klaas Jan P.; Pérez Gallent, Elena; Koper, Marc T. M.
- Journal of Electroanalytical Chemistry, Vol. 716
How to Conceptualize Catalytic Cycles? The Energetic Span Model
journal, February 2011
- Kozuch, Sebastian; Shaik, Sason
- Accounts of Chemical Research, Vol. 44, Issue 2
C i s ‐glyoxal in effusive and supersonic beams. Spectroscopy, selective pumping, and c i s – t r a n s interconversion
journal, June 1987
- Butz, Kirk W.; Johnson, Jeffrey R.; Krajnovich, Douglas J.
- The Journal of Chemical Physics, Vol. 86, Issue 11
The unimolecular triple dissociation of glyoxal: transition-state structures optimized by configuration interaction and coupled cluster methods
journal, September 1989
- Scuseria, Gustavo E.; Schaefer, Henry F.
- Journal of the American Chemical Society, Vol. 111, Issue 20
The Importance of Cannizzaro-Type Reactions during Electrocatalytic Reduction of Carbon Dioxide
journal, January 2017
- Birdja, Yuvraj Y.; Koper, Marc T. M.
- Journal of the American Chemical Society, Vol. 139, Issue 5
Acetaldehyde as an Intermediate in the Electroreduction of Carbon Monoxide to Ethanol on Oxide-Derived Copper
journal, December 2015
- Bertheussen, Erlend; Verdaguer-Casadevall, Arnau; Ravasio, Davide
- Angewandte Chemie International Edition, Vol. 55, Issue 4
Generation of α-acetolactone and the acetoxyl diradical •CH2COO• in the gas phase
journal, November 1997
- Schröder, Detlef; Goldberg, Norman; Zummack, Waltraud
- International Journal of Mass Spectrometry and Ion Processes, Vol. 165-166
Works referencing / citing this record:
Advances and challenges in modeling solvated reaction mechanisms for renewable fuels and chemicals
journal, August 2019
- Basdogan, Yasemin; Maldonado, Alex M.; Keith, John A.
- WIREs Computational Molecular Science, Vol. 10, Issue 2
Electrochemical Scanning Probe Microscopies in Electrocatalysis
journal, November 2018
- Liang, Yunchang; Pfisterer, Jonas H. K.; McLaughlin, David
- Small Methods, Vol. 3, Issue 8
Nanostructured Copper-Based Electrocatalysts for CO 2 Reduction
journal, July 2018
- Gu, Zhengxiang; Shen, Hao; Shang, Longmei
- Small Methods, Vol. 2, Issue 11
Hybrid Cu 0 and Cu x + as Atomic Interfaces Promote High‐Selectivity Conversion of CO 2 to C 2 H 5 OH at Low Potential
journal, March 2020
- Bai, Xiaowan; Li, Qiang; Shi, Li
- Small, Vol. 16, Issue 12
Computational and experimental demonstrations of one-pot tandem catalysis for electrochemical carbon dioxide reduction to methane
journal, July 2019
- Zhang, Haochen; Chang, Xiaoxia; Chen, Jingguang G.
- Nature Communications, Vol. 10, Issue 1
Advances and challenges in electrochemical CO 2 reduction processes: an engineering and design perspective looking beyond new catalyst materials
journal, January 2020
- Garg, Sahil; Li, Mengran; Weber, Adam Z.
- Journal of Materials Chemistry A, Vol. 8, Issue 4
Evidence for product-specific active sites on oxide-derived Cu catalysts for electrochemical CO2 reduction
journal, December 2018
- Lum, Yanwei; Ager, Joel W.
- Nature Catalysis, Vol. 2, Issue 1
Formation of carbon–nitrogen bonds in carbon monoxide electrolysis
journal, August 2019
- Jouny, Matthew; Lv, Jing-Jing; Cheng, Tao
- Nature Chemistry, Vol. 11, Issue 9
pH effects on the electrochemical reduction of CO(2) towards C2 products on stepped copper
journal, January 2019
- Liu, Xinyan; Schlexer, Philomena; Xiao, Jianping
- Nature Communications, Vol. 10, Issue 1
Emerging Carbon‐Based Heterogeneous Catalysts for Electrochemical Reduction of Carbon Dioxide into Value‐Added Chemicals
journal, December 2018
- Wu, Jingjie; Sharifi, Tiva; Gao, Ying
- Advanced Materials, Vol. 31, Issue 13
Electrochemical Carbon Dioxide Splitting
journal, February 2019
- Xie, Jiafang; Huang, Yiyin; Wu, Maoxiang
- ChemElectroChem, Vol. 6, Issue 6
Selective reduction of CO to acetaldehyde with CuAg electrocatalysts
journal, January 2020
- Wang, Lei; Higgins, Drew C.; Ji, Yongfei
- Proceedings of the National Academy of Sciences, Vol. 117, Issue 23
Reactivity Determinants in Electrodeposited Cu Foams for Electrochemical CO 2 Reduction
journal, August 2018
- Klingan, Katharina; Kottakkat, Tintula; Jovanov, Zarko P.
- ChemSusChem, Vol. 11, Issue 19
Linkage Effect in the Heterogenization of Cobalt Complexes by Doped Graphene for Electrocatalytic CO 2 Reduction
journal, September 2019
- Wang, Jiong; Huang, Xiang; Xi, Shibo
- Angewandte Chemie International Edition, Vol. 58, Issue 38
Highly Selective Production of Ethylene by the Electroreduction of Carbon Monoxide
journal, December 2019
- Chen, Ruixue; Su, Hai‐Yan; Liu, Deyu
- Angewandte Chemie, Vol. 132, Issue 1
Atomic Layer Deposition of ZnO on CuO Enables Selective and Efficient Electroreduction of Carbon Dioxide to Liquid Fuels
journal, September 2019
- Ren, Dan; Gao, Jing; Pan, Linfeng
- Angewandte Chemie, Vol. 131, Issue 42
Structure‐Sensitivity and Electrolyte Effects in CO 2 Electroreduction: From Model Studies to Applications
journal, June 2019
- Sebastián‐Pascual, Paula; Mezzavilla, Stefano; Stephens, Ifan E. L.
- ChemCatChem, Vol. 11, Issue 16
Surface strategies for catalytic CO 2 reduction: from two-dimensional materials to nanoclusters to single atoms
journal, January 2019
- Wang, Liming; Chen, Wenlong; Zhang, Doudou
- Chemical Society Reviews, Vol. 48, Issue 21
Establishing new scaling relations on two-dimensional MXenes for CO 2 electroreduction
journal, January 2018
- Handoko, Albertus D.; Khoo, Khoong Hong; Tan, Teck Leong
- Journal of Materials Chemistry A, Vol. 6, Issue 44
Changing the Product Selectivity for Electrocatalysis of CO 2 Reduction Reaction on Plated Cu Electrodes
journal, October 2019
- Li, Hong; Qin, Xianxian; Jiang, Tianwen
- ChemCatChem, Vol. 11, Issue 24
A Disquisition on the Active Sites of Heterogeneous Catalysts for Electrochemical Reduction of CO 2 to Value‐Added Chemicals and Fuel
journal, November 2019
- Daiyan, Rahman; Saputera, Wibawa Hendra; Masood, Hassan
- Advanced Energy Materials, Vol. 10, Issue 11
Electrocatalytic Reduction of Gaseous CO 2 to CO on Sn/Cu‐Nanofiber‐Based Gas Diffusion Electrodes
journal, July 2019
- Ju, Wenbo; Jiang, Fuze; Ma, Huan
- Advanced Energy Materials, Vol. 9, Issue 32
Bimetallic Electrocatalysts for CO2 Reduction
journal, October 2018
- Zhu, Wenlei; Tackett, Brian M.; Chen, Jingguang G.
- Topics in Current Chemistry, Vol. 376, Issue 6
Advances and challenges in understanding the electrocatalytic conversion of carbon dioxide to fuels
journal, September 2019
- Birdja, Yuvraj Y.; Pérez-Gallent, Elena; Figueiredo, Marta C.
- Nature Energy, Vol. 4, Issue 9
Photodriven CO dimerization on Cu 2 O from an electronic-structure perspective
journal, January 2020
- Hedström, Svante; Halldin Stenlid, Joakim; Liu, Chang
- Sustainable Energy & Fuels, Vol. 4, Issue 2
A Highly Porous Copper Electrocatalyst for Carbon Dioxide Reduction
journal, October 2018
- Lv, Jing-Jing; Jouny, Matthew; Luc, Wesley
- Advanced Materials, Vol. 30, Issue 49
Surface Reconstruction of Polycrystalline Cu Electrodes in Aqueous KHCO3 Electrolyte at Potentials in the Early Stages of CO2 Reduction
journal, April 2018
- Kim, Youn-Geun; Baricuatro, Jack H.; Soriaga, Manuel P.
- Electrocatalysis, Vol. 9, Issue 4
Toward Computational Design of Catalysts for CO 2 Selective Reduction via Reaction Phase Diagram Analysis
journal, January 2019
- Han, Mengru; Fu, Xiaoyan; Cao, Ang
- Advanced Theory and Simulations, Vol. 2, Issue 3
Electrochemical CO 2 reduction on Cu and Au electrodes studied using in situ sum frequency generation spectroscopy
journal, January 2019
- Huang-fu, Zhi-Chao; Song, Qian-Tong; He, Yu-Han
- Physical Chemistry Chemical Physics, Vol. 21, Issue 45
Highly Selective Production of Ethylene by the Electroreduction of Carbon Monoxide
journal, December 2019
- Chen, Ruixue; Su, Hai‐Yan; Liu, Deyu
- Angewandte Chemie International Edition, Vol. 59, Issue 1
Review of two‐dimensional materials for electrochemical CO 2 reduction from a theoretical perspective
journal, April 2019
- Zhu, Xiaorong; Li, Yafei
- Wiley Interdisciplinary Reviews: Computational Molecular Science, Vol. 9, Issue 6
Carbon monoxide electroreduction as an emerging platform for carbon utilization
journal, December 2019
- Jouny, Matthew; Hutchings, Gregory S.; Jiao, Feng
- Nature Catalysis, Vol. 2, Issue 12
Metal-free graphdiyne doped with sp-hybridized boron and nitrogen atoms at acetylenic sites for high-efficiency electroreduction of CO 2 to CH 4 and C 2 H 4
journal, January 2019
- Zhao, Jia; Chen, Zhe; Zhao, Jingxiang
- Journal of Materials Chemistry A, Vol. 7, Issue 8
Atomic Layer Deposition of ZnO on CuO Enables Selective and Efficient Electroreduction of Carbon Dioxide to Liquid Fuels
journal, September 2019
- Ren, Dan; Gao, Jing; Pan, Linfeng
- Angewandte Chemie International Edition, Vol. 58, Issue 42
Linkage Effect in the Heterogenization of Cobalt Complexes by Doped Graphene for Electrocatalytic CO 2 Reduction
journal, August 2019
- Wang, Jiong; Huang, Xiang; Xi, Shibo
- Angewandte Chemie, Vol. 131, Issue 38
High-rate electroreduction of carbon monoxide to multi-carbon products
journal, August 2018
- Jouny, Matthew; Luc, Wesley; Jiao, Feng
- Nature Catalysis, Vol. 1, Issue 10
Reaction intermediates during operando electrocatalysis identified from full solvent quantum mechanics molecular dynamics
journal, March 2019
- Cheng, Tao; Fortunelli, Alessandro; Goddard, William A.
- Proceedings of the National Academy of Sciences, Vol. 116, Issue 16
Heterogeneous catalysts for catalytic CO2 conversion into value-added chemicals
journal, March 2019
- Whang, Ho Seok; Lim, Jinkyu; Choi, Min Suk
- BMC Chemical Engineering, Vol. 1, Issue 1
Mechanism and kinetics for both thermal and electrochemical reduction of N 2 catalysed by Ru(0001) based on quantum mechanics
journal, January 2019
- Chen, Liang-Yu; Kuo, Tung-Chun; Hong, Zih-Siang
- Physical Chemistry Chemical Physics, Vol. 21, Issue 32
Silicon-doped graphene edges: an efficient metal-free catalyst for the reduction of CO 2 into methanol and ethanol
journal, January 2019
- Mao, Xin; Kour, Gurpreet; Zhang, Lei
- Catalysis Science & Technology, Vol. 9, Issue 23
Reduction of carbon dioxide on photoexcited nanoparticles of VIII group metals
journal, January 2019
- Dai, Xinyan; Sun, Yugang
- Nanoscale, Vol. 11, Issue 36
Regulating C–C coupling in thermocatalytic and electrocatalytic CO x conversion based on surface science
journal, January 2019
- Jiang, Yawen; Long, Ran; Xiong, Yujie
- Chemical Science, Vol. 10, Issue 31
Promises of Main Group Metal–Based Nanostructured Materials for Electrochemical CO 2 Reduction to Formate
journal, November 2019
- Han, Na; Ding, Pan; He, Le
- Advanced Energy Materials, Vol. 10, Issue 11
Mononuclear Fe in N-doped carbon: computational elucidation of active sites for electrochemical oxygen reduction and oxygen evolution reactions
journal, January 2020
- Shang, Rui; Steinmann, Stephan N.; Xu, Bo-Qing
- Catalysis Science & Technology, Vol. 10, Issue 4
Copper adparticle enabled selective electrosynthesis of n-propanol
journal, November 2018
- Li, Jun; Che, Fanglin; Pang, Yuanjie
- Nature Communications, Vol. 9, Issue 1
Potential-Dependent CO2 Electroreduction Pathways on Cu(111) Based on an Improved Electrode/Aqueous Interface Model: Determination of the Origin of the Overpotentials
journal, October 2019
- Ou, Lihui; Zhao, Kexin
- ACS Omega, Vol. 4, Issue 17
pH effects on the electrochemical reduction of CO(2) towards C2 products on stepped copper
journal, January 2019
- Liu, Xinyan; Schlexer, Philomena; Xiao, Jianping
- Nature Communications, Vol. 10, Issue 1
Galvanic Replacement of Electrochemically Restructured Copper Electrodes with Gold and Its Electrocatalytic Activity for Nitrate Ion Reduction
journal, September 2018
- Balkis, Ali; Crawford, Jessica; O’Mullane, Anthony
- Nanomaterials, Vol. 8, Issue 10
Figures / Tables found in this record:

 Search WorldCat to find libraries that may hold this journal
Search WorldCat to find libraries that may hold this journal