Nanoscale in situ detection of nucleation and growth of Li electrodeposition at various current densities
Abstract
Li metal batteries can store at least ten times more energy than currently existing Li-ion batteries. However, during routine charging and discharging, Li dendrites grow on the Li metal electrode, which can lead to capacity loss by the consumption of Li salt at the surface of the Li dendrites, and be a safety hazard resulting from the potential for short-circuits. Although past efforts have provided useful information about the morphology and surface area of Li dendrite formation at the microscale, a nanoscale understanding of nucleation and growth of Li nanoparticle electrodeposition is still elusive. In this study, using a new electrochemical cell for transmission mode grazing incidence small angle X-ray scattering, we obtained, for the first time, the primary nucleus size of Li nanoparticles, their size evolution and their fractal structures at various current densities and in real-time. The measured average radius of gyration, Rg, at current densities of 0.1, 0.5, and 2.0 mA cm-2 is 5.4 ± 0.4, 4.5 ± 0.3, and 3.5 ± 0.3 nm, respectively. This variation in size with current density is noteworthy when recognizing that the surface area-to-volume ratio of the Li nanoparticles is 3.7 times higher at 2.0 mA cm-2 than at 0.1 mAmore »
- Authors:
-
- Washington Univ., St. Louis, MO (United States). Dept. of Energy, Environmental and Chemical Engineering
- Argonne National Lab. (ANL), Argonne, IL (United States). X-ray Science Division
- Seoul National Univ. (Korea, Republic of). Program in Nano Science and Technology, Graduate School of Convergence Science and Technology
- Publication Date:
- Research Org.:
- Argonne National Lab. (ANL), Argonne, IL (United States)
- Sponsoring Org.:
- USDOE Office of Science (SC), Basic Energy Sciences (BES); National Science Foundation (NSF)
- OSTI Identifier:
- 1460946
- Grant/Contract Number:
- AC02-06CH11357
- Resource Type:
- Accepted Manuscript
- Journal Name:
- Journal of Materials Chemistry. A
- Additional Journal Information:
- Journal Volume: 6; Journal Issue: 11; Journal ID: ISSN 2050-7488
- Publisher:
- Royal Society of Chemistry
- Country of Publication:
- United States
- Language:
- English
- Subject:
- 25 ENERGY STORAGE; MRI; cells; dendrite growth; deposition; interface; ionic-liquid; lithium metal anodes; sulfur batteries
Citation Formats
Jung, Haesung, Lee, Byeongdu, Lengyel, Miklos, Axelbaum, Richard, Yoo, Jeeyoung, Kim, Youn Sang, and Jun, Young-Shin. Nanoscale in situ detection of nucleation and growth of Li electrodeposition at various current densities. United States: N. p., 2018.
Web. doi:10.1039/C8TA00343B.
Jung, Haesung, Lee, Byeongdu, Lengyel, Miklos, Axelbaum, Richard, Yoo, Jeeyoung, Kim, Youn Sang, & Jun, Young-Shin. Nanoscale in situ detection of nucleation and growth of Li electrodeposition at various current densities. United States. https://doi.org/10.1039/C8TA00343B
Jung, Haesung, Lee, Byeongdu, Lengyel, Miklos, Axelbaum, Richard, Yoo, Jeeyoung, Kim, Youn Sang, and Jun, Young-Shin. Fri .
"Nanoscale in situ detection of nucleation and growth of Li electrodeposition at various current densities". United States. https://doi.org/10.1039/C8TA00343B. https://www.osti.gov/servlets/purl/1460946.
@article{osti_1460946,
title = {Nanoscale in situ detection of nucleation and growth of Li electrodeposition at various current densities},
author = {Jung, Haesung and Lee, Byeongdu and Lengyel, Miklos and Axelbaum, Richard and Yoo, Jeeyoung and Kim, Youn Sang and Jun, Young-Shin},
abstractNote = {Li metal batteries can store at least ten times more energy than currently existing Li-ion batteries. However, during routine charging and discharging, Li dendrites grow on the Li metal electrode, which can lead to capacity loss by the consumption of Li salt at the surface of the Li dendrites, and be a safety hazard resulting from the potential for short-circuits. Although past efforts have provided useful information about the morphology and surface area of Li dendrite formation at the microscale, a nanoscale understanding of nucleation and growth of Li nanoparticle electrodeposition is still elusive. In this study, using a new electrochemical cell for transmission mode grazing incidence small angle X-ray scattering, we obtained, for the first time, the primary nucleus size of Li nanoparticles, their size evolution and their fractal structures at various current densities and in real-time. The measured average radius of gyration, Rg, at current densities of 0.1, 0.5, and 2.0 mA cm-2 is 5.4 ± 0.4, 4.5 ± 0.3, and 3.5 ± 0.3 nm, respectively. This variation in size with current density is noteworthy when recognizing that the surface area-to-volume ratio of the Li nanoparticles is 3.7 times higher at 2.0 mA cm-2 than at 0.1 mA cm-2. We also compared a hierarchical fractal structure of Li particles from the nanometer to micrometer scale. Our findings illuminate the role of overpotential in the reactive surface area of Li dendrites at the nanoscale, and provide a novel research platform for suppressing Li dendrite formation in Li metal battery systems.},
doi = {10.1039/C8TA00343B},
journal = {Journal of Materials Chemistry. A},
number = 11,
volume = 6,
place = {United States},
year = {Fri Feb 02 00:00:00 EST 2018},
month = {Fri Feb 02 00:00:00 EST 2018}
}
Web of Science
Figures / Tables:
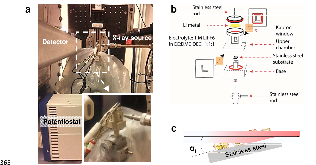 Fig. 1: Experimental setup for in situ transmission GISAXS. (a) Newly developed Li electrodeposition cell operated in beamline 12-ID-B, Advanced Photon Sources (APS), Argonne National Laboratory (ANL), IL. (b) Schematic cell design for Li electrodeposition. (c) The measurement of Li electrodeposition at an electrolyte-electrode interface using in situ time resolvedmore »
Fig. 1: Experimental setup for in situ transmission GISAXS. (a) Newly developed Li electrodeposition cell operated in beamline 12-ID-B, Advanced Photon Sources (APS), Argonne National Laboratory (ANL), IL. (b) Schematic cell design for Li electrodeposition. (c) The measurement of Li electrodeposition at an electrolyte-electrode interface using in situ time resolvedmore »
Works referenced in this record:
In situ NMR observation of the formation of metallic lithium microstructures in lithium batteries
journal, May 2010
- Bhattacharyya, Rangeet; Key, Baris; Chen, Hailong
- Nature Materials, Vol. 9, Issue 6
Direct In Situ Observation and Numerical Simulations of Non-Shrinking-Core Behavior in an MCMB Graphite Composite Electrode
journal, January 2012
- Harris, Stephen J.; Rahani, Ehsan Kabiri; Shenoy, Vivek B.
- Journal of The Electrochemical Society, Vol. 159, Issue 9
Prospects for Dendrite-Free Cycling of Li Metal Batteries
journal, January 2015
- Chen, Qing; Geng, Ke; Sieradzki, K.
- Journal of The Electrochemical Society, Vol. 162, Issue 10
Ionic-Liquid-Nanoparticle Hybrid Electrolytes: Applications in Lithium Metal Batteries
journal, November 2013
- Lu, Yingying; Korf, Kevin; Kambe, Yu
- Angewandte Chemie International Edition, Vol. 53, Issue 2
Ultrathin Two-Dimensional Atomic Crystals as Stable Interfacial Layer for Improvement of Lithium Metal Anode
journal, September 2014
- Yan, Kai; Lee, Hyun-Wook; Gao, Teng
- Nano Letters, Vol. 14, Issue 10
Controlled Lithium Dendrite Growth by a Synergistic Effect of Multilayered Graphene Coating and an Electrolyte Additive
journal, April 2015
- Kim, Joo-Seong; Kim, Dae Woo; Jung, Hee Tae
- Chemistry of Materials, Vol. 27, Issue 8
Anode-Free Rechargeable Lithium Metal Batteries
journal, August 2016
- Qian, Jiangfeng; Adams, Brian D.; Zheng, Jianming
- Advanced Functional Materials, Vol. 26, Issue 39
Lithium-coated polymeric matrix as a minimum volume-change and dendrite-free lithium metal anode
journal, March 2016
- Liu, Yayuan; Lin, Dingchang; Liang, Zheng
- Nature Communications, Vol. 7, Issue 1
Optical observation of Li dendrite growth in ionic liquid
journal, June 2013
- Nishida, T.; Nishikawa, K.; Rosso, M.
- Electrochimica Acta, Vol. 100
Nanoscale Nucleation and Growth of Electrodeposited Lithium Metal
journal, January 2017
- Pei, Allen; Zheng, Guangyuan; Shi, Feifei
- Nano Letters, Vol. 17, Issue 2
Designing Safe Electrolyte Systems for a High-Stability Lithium-Sulfur Battery
journal, January 2018
- Chen, Wei; Lei, Tianyu; Wu, Chunyang
- Advanced Energy Materials, Vol. 8, Issue 10
Electrochemical aspects of the generation of ramified metallic electrodeposits
journal, December 1990
- Chazalviel, J. -N.
- Physical Review A, Vol. 42, Issue 12
Lithiophilic Sites in Doped Graphene Guide Uniform Lithium Nucleation for Dendrite-Free Lithium Metal Anodes
journal, May 2017
- Zhang, Rui; Chen, Xiao-Ru; Chen, Xiang
- Angewandte Chemie International Edition, Vol. 56, Issue 27
Nanoscale Imaging of Fundamental Li Battery Chemistry: Solid-Electrolyte Interphase Formation and Preferential Growth of Lithium Metal Nanoclusters
journal, February 2015
- Sacci, Robert L.; Black, Jennifer M.; Balke, Nina
- Nano Letters, Vol. 15, Issue 3
Effects of current densities on the lithium plating morphology at a lithium phosphorus oxynitride glass electrolyte/copper thin film interface
journal, July 2013
- Sagane, Fumihiro; Ikeda, Ken-ichi; Okita, Kengo
- Journal of Power Sources, Vol. 233
Magnetosome-like ferrimagnetic iron oxide nanocubes for highly sensitive MRI of single cells and transplanted pancreatic islets
journal, January 2011
- Lee, N.; Kim, H.; Choi, S. H.
- Proceedings of the National Academy of Sciences, Vol. 108, Issue 7
In Situ Observations of Nanoparticle Early Development Kinetics at Mineral−Water Interfaces
journal, November 2010
- Jun, Young-Shin; Lee, Byeongdu; Waychunas, Glenn A.
- Environmental Science & Technology, Vol. 44, Issue 21
Columnar Lithium Metal Anodes
journal, September 2017
- Zhang, Xue-Qiang; Chen, Xiang; Xu, Rui
- Angewandte Chemie International Edition, Vol. 56, Issue 45
Dendrite-Free Lithium Deposition via Self-Healing Electrostatic Shield Mechanism
journal, March 2013
- Ding, Fei; Xu, Wu; Graff, Gordon L.
- Journal of the American Chemical Society, Vol. 135, Issue 11, p. 4450-4456
Three-dimensional characterization of electrodeposited lithium microstructures using synchrotron X-ray phase contrast imaging
journal, January 2015
- Eastwood, David S.; Bayley, Paul M.; Chang, Hee Jung
- Chemical Communications, Vol. 51, Issue 2
7Li MRI of Li batteries reveals location of microstructural lithium
journal, February 2012
- Chandrashekar, S.; Trease, Nicole M.; Chang, Hee Jung
- Nature Materials, Vol. 11, Issue 4
Design principles for electrolytes and interfaces for stable lithium-metal batteries
journal, September 2016
- Tikekar, Mukul D.; Choudhury, Snehashis; Tu, Zhengyuan
- Nature Energy, Vol. 1, Issue 9
The critical role of lithium nitrate in the gas evolution of lithium–sulfur batteries
journal, January 2016
- Jozwiuk, Anna; Berkes, Balázs B.; Weiß, Thomas
- Energy & Environmental Science, Vol. 9, Issue 8
Heterogeneous Nucleation and Growth of Lithium Electrodeposits on Negative Electrodes
journal, January 2013
- Ely, David R.; García, R. Edwin
- Journal of The Electrochemical Society, Vol. 160, Issue 4
Grazing-incidence transmission small angle X-ray scattering from thin films of block copolymers
journal, February 2013
- Mahadevapuram, Nikhila; Strzalka, Joseph; Stein, Gila E.
- Journal of Polymer Science Part B: Polymer Physics, Vol. 51, Issue 7
Li–O2 and Li–S batteries with high energy storage
journal, January 2012
- Bruce, Peter G.; Freunberger, Stefan A.; Hardwick, Laurence J.
- Nature Materials, Vol. 11, Issue 1, p. 19-29
A review of lithium deposition in lithium-ion and lithium metal secondary batteries
journal, May 2014
- Li, Zhe; Huang, Jun; Yann Liaw, Bor
- Journal of Power Sources, Vol. 254
Lithium metal anodes for rechargeable batteries
journal, January 2014
- Xu, Wu; Wang, Jiulin; Ding, Fei
- Energy Environ. Sci., Vol. 7, Issue 2
Dendritic growth mechanisms in lithium/polymer cells
journal, September 1999
- Brissot, C.; Rosso, M.; Chazalviel, J. -N.
- Journal of Power Sources, Vol. 81-82
Transition of lithium growth mechanisms in liquid electrolytes
journal, January 2016
- Bai, Peng; Li, Ju; Brushett, Fikile R.
- Energy & Environmental Science, Vol. 9, Issue 10
Observation and Quantification of Nanoscale Processes in Lithium Batteries by Operando Electrochemical (S)TEM
journal, February 2015
- Mehdi, B. L.; Qian, J.; Nasybulin, E.
- Nano Letters, Vol. 15, Issue 3
Electroless Formation of Hybrid Lithium Anodes for Fast Interfacial Ion Transport
journal, September 2017
- Choudhury, Snehashis; Tu, Zhengyuan; Stalin, Sanjuna
- Angewandte Chemie International Edition, Vol. 56, Issue 42
Branched fractal patterns in non-equilibrium electrochemical deposition from oscillatory nucleation and growth
journal, November 1997
- Fleury, Vincent
- Nature, Vol. 390, Issue 6656
A highly ordered nanostructured carbon–sulphur cathode for lithium–sulphur batteries
journal, May 2009
- Ji, Xiulei; Lee, Kyu Tae; Nazar, Linda F.
- Nature Materials, Vol. 8, Issue 6, p. 500-506
Detection of subsurface structures underneath dendrites formed on cycled lithium metal electrodes
journal, November 2013
- Harry, Katherine J.; Hallinan, Daniel T.; Parkinson, Dilworth Y.
- Nature Materials, Vol. 13, Issue 1
Interconnected hollow carbon nanospheres for stable lithium metal anodes
journal, July 2014
- Zheng, Guangyuan; Lee, Seok Woo; Liang, Zheng
- Nature Nanotechnology, Vol. 9, Issue 8
High rate and stable cycling of lithium metal anode
journal, February 2015
- Qian, Jiangfeng; Henderson, Wesley A.; Xu, Wu
- Nature Communications, Vol. 6, Issue 1
The interplay between solid electrolyte interface (SEI) and dendritic lithium growth
journal, October 2017
- Wu, Bingbin; Lochala, Joshua; Taverne, Tyler
- Nano Energy, Vol. 40
Lithiophilic Sites in Doped Graphene Guide Uniform Lithium Nucleation for Dendrite-Free Lithium Metal Anodes
journal, May 2017
- Zhang, Rui; Chen, Xiao-Ru; Chen, Xiang
- Angewandte Chemie, Vol. 129, Issue 27
Electroless Formation of Hybrid Lithium Anodes for Fast Interfacial Ion Transport
journal, September 2017
- Choudhury, Snehashis; Tu, Zhengyuan; Stalin, Sanjuna
- Angewandte Chemie, Vol. 129, Issue 42
Columnar Lithium Metal Anodes
journal, September 2017
- Zhang, Xue-Qiang; Chen, Xiang; Xu, Rui
- Angewandte Chemie, Vol. 129, Issue 45
The critical role of lithium nitrate in the gas evolution of lithium–sulfur batteries
text, January 2016
- Jozwiuk, Anna; Berkes, Balázs B.; Weiß, Thomas
- Karlsruhe
Works referencing / citing this record:
Favorable lithium deposition behaviors on flexible carbon microtube skeleton enable a high-performance lithium metal anode
journal, January 2018
- Sun, Changzhi; Wu, Tian; Wang, Jianing
- Journal of Materials Chemistry A, Vol. 6, Issue 39
An acetylene black modified gel polymer electrolyte for high-performance lithium–sulfur batteries
journal, January 2019
- Yang, Dezhi; He, Liang; Liu, Yu
- Journal of Materials Chemistry A, Vol. 7, Issue 22
Figures / Tables found in this record:

 Search WorldCat to find libraries that may hold this journal
Search WorldCat to find libraries that may hold this journal