Highly Active, Durable Dispersed Iridium Nanocatalysts for PEM Water Electrolyzers
Abstract
One of the primary challenges for proton exchange membrane (PEM) electrolyzers is the sluggish kinetics of the oxygen evolution reaction (OER) at the anode, which requires the use of precious metals or metal oxides, such as iridium (Ir) or iridium oxide (IrOx), as the OER catalyst. This study introduces a one-pot surfactant-free polyol reduction method to disperse iridium nanoparticles on a tungsten doped titanium oxide (WxTi1-xO2) support. The polyol reduction approach for the Ir/WxTi1-xO2 catalyst synthesis was systematically investigated to determine the influence of synthesis parameters on the catalysts’ physical properties, and its electrochemical activity and durability. The most promising synthesized catalyst with 38 wt% Ir (Ir38%/WxTi1-xO2) demonstrated five times higher mass activity than an Ir-black baseline (the industry standard catalyst) based on rotating-disk electrode (RDE) studies. When tested in a real water electrolyzer system, the synthesized catalyst enabled the Ir loading to be lowered by an order of magnitude while retaining a similar electrolyzer performance found for the baseline Ir-black catalyst. In conclusion, the Ir38%/WxTi1-xO2 catalyst also demonstrated remarkable stability, e.g., only small voltage (<20 mV) increase was observed during a 1200-hour durability test at a constant current density of 1500 mA/cm2.
- Authors:
-
- Giner Inc. Newton, MA (United States)
- Oak Ridge National Lab. (ORNL), Oak Ridge, TN (United States). Center for Nanophase Materials Science (CNMS)
- Publication Date:
- Research Org.:
- Oak Ridge National Lab. (ORNL), Oak Ridge, TN (United States)
- Sponsoring Org.:
- USDOE Office of Energy Efficiency and Renewable Energy (EERE); USDOE Office of Nuclear Energy (NE), Fuel Cycle Technologies (NE-5)
- OSTI Identifier:
- 1460216
- Grant/Contract Number:
- AC05-00OR22725; SC0007471
- Resource Type:
- Accepted Manuscript
- Journal Name:
- Journal of the Electrochemical Society
- Additional Journal Information:
- Journal Volume: 165; Journal Issue: 2; Journal ID: ISSN 0013-4651
- Publisher:
- The Electrochemical Society
- Country of Publication:
- United States
- Language:
- English
- Subject:
- 37 INORGANIC, ORGANIC, PHYSICAL, AND ANALYTICAL CHEMISTRY; Oxygen Evolution Catalyst; Proton Exchange Membrane; Water Electrolyzer
Citation Formats
Zhao, Shuai, Stocks, Allison, Rasimick, Brian, More, Karren, and Xu, Hui. Highly Active, Durable Dispersed Iridium Nanocatalysts for PEM Water Electrolyzers. United States: N. p., 2018.
Web. doi:10.1149/2.0981802jes.
Zhao, Shuai, Stocks, Allison, Rasimick, Brian, More, Karren, & Xu, Hui. Highly Active, Durable Dispersed Iridium Nanocatalysts for PEM Water Electrolyzers. United States. https://doi.org/10.1149/2.0981802jes
Zhao, Shuai, Stocks, Allison, Rasimick, Brian, More, Karren, and Xu, Hui. Mon .
"Highly Active, Durable Dispersed Iridium Nanocatalysts for PEM Water Electrolyzers". United States. https://doi.org/10.1149/2.0981802jes. https://www.osti.gov/servlets/purl/1460216.
@article{osti_1460216,
title = {Highly Active, Durable Dispersed Iridium Nanocatalysts for PEM Water Electrolyzers},
author = {Zhao, Shuai and Stocks, Allison and Rasimick, Brian and More, Karren and Xu, Hui},
abstractNote = {One of the primary challenges for proton exchange membrane (PEM) electrolyzers is the sluggish kinetics of the oxygen evolution reaction (OER) at the anode, which requires the use of precious metals or metal oxides, such as iridium (Ir) or iridium oxide (IrOx), as the OER catalyst. This study introduces a one-pot surfactant-free polyol reduction method to disperse iridium nanoparticles on a tungsten doped titanium oxide (WxTi1-xO2) support. The polyol reduction approach for the Ir/WxTi1-xO2 catalyst synthesis was systematically investigated to determine the influence of synthesis parameters on the catalysts’ physical properties, and its electrochemical activity and durability. The most promising synthesized catalyst with 38 wt% Ir (Ir38%/WxTi1-xO2) demonstrated five times higher mass activity than an Ir-black baseline (the industry standard catalyst) based on rotating-disk electrode (RDE) studies. When tested in a real water electrolyzer system, the synthesized catalyst enabled the Ir loading to be lowered by an order of magnitude while retaining a similar electrolyzer performance found for the baseline Ir-black catalyst. In conclusion, the Ir38%/WxTi1-xO2 catalyst also demonstrated remarkable stability, e.g., only small voltage (<20 mV) increase was observed during a 1200-hour durability test at a constant current density of 1500 mA/cm2.},
doi = {10.1149/2.0981802jes},
journal = {Journal of the Electrochemical Society},
number = 2,
volume = 165,
place = {United States},
year = {Mon Jan 01 00:00:00 EST 2018},
month = {Mon Jan 01 00:00:00 EST 2018}
}
Web of Science
Figures / Tables:
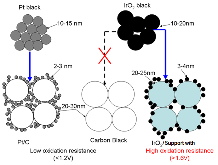 Figure 1.: Schematic of catalyst support comparison for ORR in fuel cells and OER in electrolysis cells.
Figure 1.: Schematic of catalyst support comparison for ORR in fuel cells and OER in electrolysis cells.
Works referenced in this record:
Multimetallic Electrocatalysts of Pt, Ru, and Ir Supported on Anatase and Rutile TiO[sub 2] for Oxygen Evolution in an Acid Environment
journal, January 2011
- Fuentes, Roderick E.; Farell, Jake; Weidner, John W.
- Electrochemical and Solid-State Letters, Vol. 14, Issue 3
Determining the Electrochemically Active Area of IrOx Powder Catalysts in an Operating Proton Exchange Membrane Electrolyzer
journal, September 2015
- Zhao, S.; Yu, H.; Maric, R.
- ECS Transactions, Vol. 69, Issue 17
The influence of Pt on the electrooxidation behaviour of carbon in phosphoric acid
journal, December 1992
- Passalacqua, E.; Antonucci, P. L.; Vivaldi, M.
- Electrochimica Acta, Vol. 37, Issue 15
Calculating the Electrochemically Active Surface Area of Iridium Oxide in Operating Proton Exchange Membrane Electrolyzers
journal, January 2015
- Zhao, Shuai; Yu, Haoran; Maric, Radenka
- Journal of The Electrochemical Society, Vol. 162, Issue 12
Carbon/titanium oxide supported bimetallic platinum/iridium nanocomposites as bifunctional electrocatalysts for lithium-air batteries
journal, February 2016
- Zahoor, Awan; Christy, Maria; Kim, Yongbin
- Journal of Solid State Electrochemistry, Vol. 20, Issue 5
Electrocatalytic Oxygen Evolution Reaction (OER) on Ru, Ir, and Pt Catalysts: A Comparative Study of Nanoparticles and Bulk Materials
journal, July 2012
- Reier, Tobias; Oezaslan, Mehtap; Strasser, Peter
- ACS Catalysis, Vol. 2, Issue 8
The role of titanium nitride supports for single-atom platinum-based catalysts in fuel cell technology
journal, January 2012
- Zhang, Ren-Qin; Lee, Tae-Hun; Yu, Byung-Deok
- Physical Chemistry Chemical Physics, Vol. 14, Issue 48
Titanium carbide and carbonitride electrocatalyst supports: modifying Pt–Ti interface properties by electrochemical potential cycling
journal, January 2014
- Roca-Ayats, M.; García, G.; Peña, M. A.
- J. Mater. Chem. A, Vol. 2, Issue 44
In Situ Observation of Surface Species on Iridium Oxide Nanoparticles during the Oxygen Evolution Reaction
journal, May 2014
- Sanchez Casalongue, Hernan G.; Ng, May Ling; Kaya, Sarp
- Angewandte Chemie International Edition, Vol. 53, Issue 28
A Molecular Approach to Self-Supported Cobalt-Substituted ZnO Materials as Remarkably Stable Electrocatalysts for Water Oxidation
journal, April 2014
- Pfrommer, Johannes; Lublow, Michael; Azarpira, Anahita
- Angewandte Chemie, Vol. 126, Issue 20
Nanosize Ti–W Mixed Oxides: Effect of Doping Level in the Photocatalytic Degradation of Toluene Using Sunlight-Type Excitation
journal, November 2002
- Fuerte, A.; Hernández-Alonso, M. D.; Maira, A. J.
- Journal of Catalysis, Vol. 212, Issue 1
Size-Selected Synthesis of PtRu Nano-Catalysts: Reaction and Size Control Mechanism
journal, June 2004
- Bock, Christina; Paquet, Chantal; Couillard, Martin
- Journal of the American Chemical Society, Vol. 126, Issue 25
On the oxygen evolution reaction at IrO 2 -SnO 2 mixed-oxide electrodes
journal, November 2014
- Ferro, Sergio; Rosestolato, Davide; Martínez-Huitle, Carlos Alberto
- Electrochimica Acta, Vol. 146
Characterization of Vulcan Electrochemically Oxidized under Simulated PEM Fuel Cell Conditions
journal, January 2004
- Kangasniemi, K. H.; Condit, D. A.; Jarvi, T. D.
- Journal of The Electrochemical Society, Vol. 151, Issue 4
Synthesis and Electrochemical Characterization of Uniformly-Dispersed High Loading Pt Nanoparticles on Sonochemically-Treated Carbon Nanotubes
journal, December 2004
- Xing, Yangchuan
- The Journal of Physical Chemistry B, Vol. 108, Issue 50
Effect of Dopants on Grain Coalescence and Oxygen Mobility in Nanostructured Titania Anatase and Rutile
journal, January 2003
- Guidi, V.; Carotta, M. C.; Ferroni, M.
- The Journal of Physical Chemistry B, Vol. 107, Issue 1
Oxide-Supported IrNiO x Core-Shell Particles as Efficient, Cost-Effective, and Stable Catalysts for Electrochemical Water Splitting
journal, January 2015
- Nong, Hong Nhan; Oh, Hyung-Suk; Reier, Tobias
- Angewandte Chemie International Edition, Vol. 54, Issue 10
Metal–Support Interactions between Nanosized Pt and Metal Oxides (WO 3 and TiO 2 ) Studied Using X-ray Photoelectron Spectroscopy
journal, September 2011
- Lewera, Adam; Timperman, Laure; Roguska, Agata
- The Journal of Physical Chemistry C, Vol. 115, Issue 41
Increasing Pt oxygen reduction reaction activity and durability with a carbon-doped TiO2 nanocoating catalyst support
journal, January 2012
- Huang, Kan; Sasaki, Kotaro; Adzic, Radoslav R.
- Journal of Materials Chemistry, Vol. 22, Issue 33
Stability and Activity of Pt/ITO Electrocatalyst for Oxygen Reduction Reaction in Alkaline Media
journal, March 2015
- Zhao, Shuai; Wangstrom, Abbey E.; Liu, Ying
- Electrochimica Acta, Vol. 157
Nanostructured Ti−W Mixed-Metal Oxides: Structural and Electronic Properties
journal, April 2005
- Fernández-García, M.; Martínez-Arias, A.; Fuerte, A.
- The Journal of Physical Chemistry B, Vol. 109, Issue 13
Nanoscale conductive niobium oxides made through low temperature phase transformation for electrocatalyst support
journal, January 2014
- Huang, Kan; Li, Yunfeng; Yan, Litao
- RSC Advances, Vol. 4, Issue 19
Electrocatalytic Corrosion of Carbon Support in PEMFC Cathodes
journal, January 2004
- Roen, L. M.; Paik, C. H.; Jarvi, T. D.
- Electrochemical and Solid-State Letters, Vol. 7, Issue 1
Oxide-supported Ir nanodendrites with high activity and durability for the oxygen evolution reaction in acid PEM water electrolyzers
journal, January 2015
- Oh, Hyung-Suk; Nong, Hong Nhan; Reier, Tobias
- Chemical Science, Vol. 6, Issue 6
A comprehensive review on PEM water electrolysis
journal, April 2013
- Carmo, Marcelo; Fritz, David L.; Mergel, Jürgen
- International Journal of Hydrogen Energy, Vol. 38, Issue 12, p. 4901-4934
Preparation of Tractable Platinum, Rhodium, and Ruthenium Nanoclusters with Small Particle Size in Organic Media
journal, June 2000
- Wang, Yuan; Ren, Jiawen; Deng, Kai
- Chemistry of Materials, Vol. 12, Issue 6, p. 1622-1627
Study of niobium and tantalum doped titania-supported Pt electrocatalysts for methanol oxidation and oxygen reduction reactions
journal, January 2014
- Liu, Xiaoteng; Wu, Xu; Scott, Keith
- Catal. Sci. Technol., Vol. 4, Issue 11
Oxide-Supported IrNiO x Core-Shell Particles as Efficient, Cost-Effective, and Stable Catalysts for Electrochemical Water Splitting
journal, January 2015
- Nong, Hong Nhan; Oh, Hyung-Suk; Reier, Tobias
- Angewandte Chemie, Vol. 127, Issue 10
In Situ Observation of Surface Species on Iridium Oxide Nanoparticles during the Oxygen Evolution Reaction
journal, May 2014
- Sanchez Casalongue, Hernan G.; Ng, May Ling; Kaya, Sarp
- Angewandte Chemie, Vol. 126, Issue 28
Divide and conquer: neuroevolution for multiclass classification
conference, July 2018
- McDonnell, Tyler; Andoni, Sari; Bonab, Elmira
- GECCO '18: Genetic and Evolutionary Computation Conference, Proceedings of the Genetic and Evolutionary Computation Conference
Oxide-supported Ir nanodendrites with high activity and durability for the oxygen evolution reaction in acid PEM water electrolyzers
text, January 2015
- Oh, Hyung-Suk; Nong, Hong Nhan; Reier, Tobias
- Technische Universität Berlin
Divide and conquer
journal, January 2007
- Mushtaq, Najum
- Bulletin of the Atomic Scientists, Vol. 63, Issue 1
A Molecular Approach to Self-Supported Cobalt-Substituted ZnO Materials as Remarkably Stable Electrocatalysts for Water Oxidation
journal, April 2014
- Pfrommer, Johannes; Lublow, Michael; Azarpira, Anahita
- Angewandte Chemie International Edition
Works referencing / citing this record:
Highly scattered Ir oxides on TiN as an efficient oxygen evolution reaction electrocatalyst in acidic media
journal, November 2019
- Zhang, Kaikai; Mai, Wanshan; Li, Jin
- Journal of Materials Science, Vol. 55, Issue 8
Electrolyzer Durability at Low Catalyst Loading and with Dynamic Operation
journal, January 2019
- Alia, Shaun M.; Stariha, Sarah; Borup, Rod L.
- Journal of The Electrochemical Society, Vol. 166, Issue 15
OER Catalyst Stability Investigation Using RDE Technique: A Stability Measure or an Artifact?
journal, January 2019
- El-Sayed, Hany A.; Weiß, Alexandra; Olbrich, Lorenz F.
- Journal of The Electrochemical Society, Vol. 166, Issue 8
Iridium Oxygen Evolution Activity and Durability Baselines in Rotating Disk Electrode Half-Cells
journal, January 2019
- Alia, Shaun M.; Anderson, Grace C.
- Journal of The Electrochemical Society, Vol. 166, Issue 4
Figures / Tables found in this record:

 Search WorldCat to find libraries that may hold this journal
Search WorldCat to find libraries that may hold this journal