Investigation of α-MnO2 Tunneled Structures as Model Cation Hosts for Energy Storage
Abstract
Future advances in energy storage systems rely on identification of appropriate target materials and deliberate synthesis of the target materials with control of their physiochemical properties in order to disentangling the contributions of distinct properties to the functional electrochemistry. Furthermore, this goal demands systematic inquiry using model materials that provide the opportunity for significant synthetic versatility and control. Ideally, a material family that enables direct manipulation of characteristics including composition, defects and crystallite size while remaining within the defined structural framework would be necessary. Accomplishing this through direct synthetic methods is desirable to minimize the complicating effects of secondary processing.
- Authors:
-
- Stony Brook Univ., Stony Brook, NY (United States)
- Stony Brook Univ., Stony Brook, NY (United States); Brookhaven National Lab. (BNL), Upton, NY (United States)
- Publication Date:
- Research Org.:
- Brookhaven National Laboratory (BNL), Upton, NY (United States); Energy Frontier Research Centers (EFRC) (United States). Center for Mesoscale Transport Properties (m2M)
- Sponsoring Org.:
- USDOE Office of Science (SC), Basic Energy Sciences (BES)
- OSTI Identifier:
- 1438326
- Report Number(s):
- BNL-205690-2018-JAAM
Journal ID: ISSN 0001-4842
- Grant/Contract Number:
- SC0012704
- Resource Type:
- Accepted Manuscript
- Journal Name:
- Accounts of Chemical Research
- Additional Journal Information:
- Journal Volume: 51; Journal Issue: 3; Journal ID: ISSN 0001-4842
- Publisher:
- American Chemical Society
- Country of Publication:
- United States
- Language:
- English
- Subject:
- 25 ENERGY STORAGE
Citation Formats
Housel, Lisa M., Wang, Lei, Abraham, Alyson, Huang, Jianping, Renderos, Genesis D., Quilty, Calvin D., Brady, Alexander B., Marschilok, Amy C., Takeuchi, Kenneth J., and Takeuchi, Esther S. Investigation of α-MnO2 Tunneled Structures as Model Cation Hosts for Energy Storage. United States: N. p., 2018.
Web. doi:10.1021/acs.accounts.7b00478.
Housel, Lisa M., Wang, Lei, Abraham, Alyson, Huang, Jianping, Renderos, Genesis D., Quilty, Calvin D., Brady, Alexander B., Marschilok, Amy C., Takeuchi, Kenneth J., & Takeuchi, Esther S. Investigation of α-MnO2 Tunneled Structures as Model Cation Hosts for Energy Storage. United States. https://doi.org/10.1021/acs.accounts.7b00478
Housel, Lisa M., Wang, Lei, Abraham, Alyson, Huang, Jianping, Renderos, Genesis D., Quilty, Calvin D., Brady, Alexander B., Marschilok, Amy C., Takeuchi, Kenneth J., and Takeuchi, Esther S. Mon .
"Investigation of α-MnO2 Tunneled Structures as Model Cation Hosts for Energy Storage". United States. https://doi.org/10.1021/acs.accounts.7b00478. https://www.osti.gov/servlets/purl/1438326.
@article{osti_1438326,
title = {Investigation of α-MnO2 Tunneled Structures as Model Cation Hosts for Energy Storage},
author = {Housel, Lisa M. and Wang, Lei and Abraham, Alyson and Huang, Jianping and Renderos, Genesis D. and Quilty, Calvin D. and Brady, Alexander B. and Marschilok, Amy C. and Takeuchi, Kenneth J. and Takeuchi, Esther S.},
abstractNote = {Future advances in energy storage systems rely on identification of appropriate target materials and deliberate synthesis of the target materials with control of their physiochemical properties in order to disentangling the contributions of distinct properties to the functional electrochemistry. Furthermore, this goal demands systematic inquiry using model materials that provide the opportunity for significant synthetic versatility and control. Ideally, a material family that enables direct manipulation of characteristics including composition, defects and crystallite size while remaining within the defined structural framework would be necessary. Accomplishing this through direct synthetic methods is desirable to minimize the complicating effects of secondary processing.},
doi = {10.1021/acs.accounts.7b00478},
journal = {Accounts of Chemical Research},
number = 3,
volume = 51,
place = {United States},
year = {Mon Feb 19 00:00:00 EST 2018},
month = {Mon Feb 19 00:00:00 EST 2018}
}
Web of Science
Figures / Tables:
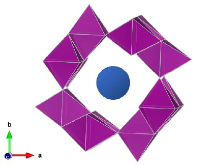 Figure 1: General structure of hollandite, with 2 x 2 tunnels and central cation.
Figure 1: General structure of hollandite, with 2 x 2 tunnels and central cation.
Works referenced in this record:
Design and Preparation of Materials for Advanced Electrochemical Storage
journal, June 2012
- Melot, Brent C.; Tarascon, J. -M.
- Accounts of Chemical Research, Vol. 46, Issue 5
Crystalline Mesoporous K 2– x Mn 8 O 16 and ε-MnO 2 by Mild Transformations of Amorphous Mesoporous Manganese Oxides and Their Enhanced Redox Properties
journal, June 2014
- Poyraz, Altug S.; Song, Wenqiao; Kriz, David
- ACS Applied Materials & Interfaces, Vol. 6, Issue 14
Structure, porosity, and redox in porous manganese oxide octahedral layer and molecular sieve materials
journal, January 2008
- Suib, Steven L.
- Journal of Materials Chemistry, Vol. 18, Issue 14
Characterization of Manganese Oxide Octahedral Molecular Sieve (M−OMS-2) Materials with Different Metal Cation Dopants
journal, February 2002
- Chen, Xiao; Shen, Yan-Fei; Suib, Steven L.
- Chemistry of Materials, Vol. 14, Issue 2
Ag1.8Mn8O16: Square Planar Coordinated Ag⊕ Ions in the Channels of a Novel Hollandite Variant
journal, November 1984
- Chang, Fung Ming; Jansen, Martin
- Angewandte Chemie International Edition in English, Vol. 23, Issue 11
Investigating the Complex Chemistry of Functional Energy Storage Systems: The Need for an Integrative, Multiscale (Molecular to Mesoscale) Perspective
journal, April 2016
- Abraham, Alyson; Housel, Lisa M.; Lininger, Christianna N.
- ACS Central Science, Vol. 2, Issue 6
M x Mn 8 O 16 (M = Ag or K) as promising cathode materials for secondary Mg based batteries: the role of the cation M
journal, January 2016
- Huang, Jianping; Poyraz, Altug S.; Takeuchi, Kenneth J.
- Chemical Communications, Vol. 52, Issue 21
The structure of K 1.33 Mn 8 O 16 and cation ordering in hollandite-type structures
journal, April 1986
- Vicat, J.; Fanchon, E.; Strobel, P.
- Acta Crystallographica Section B Structural Science, Vol. 42, Issue 2
Structural Defects of Silver Hollandite, Ag x Mn 8 O y , Nanorods: Dramatic Impact on Electrochemistry
journal, July 2015
- Wu, Lijun; Xu, Feng; Zhu, Yimei
- ACS Nano, Vol. 9, Issue 8
Topotactic Reduction of Alpha-Manganese (Di)Oxide in Nonaqueous Lithium Cells
journal, January 1991
- Ohzuku, Tsutomu
- Journal of The Electrochemical Society, Vol. 138, Issue 2
Synthesis and electrochemical properties of KMn8O16 nanorods for Lithium ion batteries
journal, January 2013
- Zheng, Hao; Feng, Chuanqi; Kim, Seung-Joo
- Electrochimica Acta, Vol. 88
Synthesis and Characterization of Silver Hollandite and Its Application in Emission Control
journal, August 2005
- Li, Liyu; King, David L.
- Chemistry of Materials, Vol. 17, Issue 17
Facile Synthesis of Ag-Hollandite Nanofibers and Their Catalytic Activity for Ethanol Selective Oxidation
journal, December 2007
- Chen, Junli; Li, Juan; Liu, Qiying
- Chinese Journal of Catalysis, Vol. 28, Issue 12
Fabrication of magnetic cryptomelane-type manganese oxide nanowires for water treatment
journal, January 2011
- Zhang, Tong; Zhang, Xiwang; Ng, Jiawei
- Chem. Commun., Vol. 47, Issue 6
Synthesis and Electrochemistry of Silver Hollandite
journal, January 2010
- Zhu, Shali; Marschilok, Amy C.; Lee, Chia-Ying
- Electrochemical and Solid-State Letters, Vol. 13, Issue 8
Synthetic Control of Composition and Crystallite Size of Silver Hollandite, Ag x Mn 8 O 16 : Impact on Electrochemistry
journal, September 2012
- Takeuchi, Kenneth J.; Yau, Shali Z.; Menard, Melissa C.
- ACS Applied Materials & Interfaces, Vol. 4, Issue 10
Tailoring the Ag + Content within the Tunnels and on the Exposed Surfaces of α-MnO 2 Nanowires: Impact on Impedance and Electrochemistry
journal, December 2016
- Zhang, Bingjie; Smith, Paul F.; Lee, Seung-Yong
- Journal of The Electrochemical Society, Vol. 164, Issue 1
Silver-Containing α-MnO 2 Nanorods: Electrochemistry in Na-Based Battery Systems
journal, September 2016
- Huang, Jianping; Poyraz, Altug S.; Lee, Seung-Yong
- ACS Applied Materials & Interfaces, Vol. 9, Issue 5
Water Oxidation Catalysis using Amorphous Manganese Oxides, Octahedral Molecular Sieves (OMS-2), and Octahedral Layered (OL-1) Manganese Oxide Structures
journal, February 2012
- Iyer, Aparna; Del-Pilar, Joselyn; King’ondu, Cecil K.
- The Journal of Physical Chemistry C, Vol. 116, Issue 10
Size-controlled synthesis and formation mechanism of manganese oxide OMS-2 nanowires under reflux conditions with KMnO 4 and inorganic acids
journal, May 2016
- Zhang, Qin; Cheng, Xiaodi; Qiu, Guohong
- Solid State Sciences, Vol. 55
Synthesis of cryptomelane type α-MnO 2 (K x Mn 8 O 16 ) cathode materials with tunable K + content: the role of tunnel cation concentration on electrochemistry
journal, January 2017
- Poyraz, Altug S.; Huang, Jianping; Pelliccione, Christopher J.
- Journal of Materials Chemistry A, Vol. 5, Issue 32
Effective recycling of manganese oxide cathodes for lithium based batteries
journal, January 2016
- Poyraz, Altug S.; Huang, Jianping; Cheng, Shaobo
- Green Chemistry, Vol. 18, Issue 11
Hydrothermal Synthesis of Manganese Oxide Nanomaterials and Their Catalytic and Electrochemical Properties
journal, September 2011
- Qiu, Guohong; Huang, Hui; Dharmarathna, Saminda
- Chemistry of Materials, Vol. 23, Issue 17
Tuning the K + Concentration in the Tunnel of OMS-2 Nanorods Leads to a Significant Enhancement of the Catalytic Activity for Benzene Oxidation
journal, November 2013
- Hou, Jingtao; Liu, Liangliang; Li, Yuanzhi
- Environmental Science & Technology, Vol. 47, Issue 23
Facile One-Step Template-Free Synthesis of Uniform Hollow Microstructures of Cryptomelane-Type Manganese Oxide K-OMS-2
journal, August 2010
- Galindo, Hugo M.; Carvajal, Yadira; Njagi, Eric
- Langmuir, Vol. 26, Issue 16
Microstructural Features of α-MnO[sub 2] Electrodes for Lithium Batteries
journal, January 1998
- Shao-Horn, Y.
- Journal of The Electrochemical Society, Vol. 145, Issue 2
Ammonia- and lithia-doped manganese dioxide for 3 V lithium batteries
journal, July 2001
- Johnson, C. S.; Thackeray, M. M.
- Journal of Power Sources, Vol. 97-98
K 0.25 Mn 2 O 4 nanofiber microclusters as high power cathode materials for rechargeable lithium batteries
journal, January 2012
- Zhang, Chaofeng; Feng, Chuanqi; Zhang, Peng
- RSC Adv., Vol. 2, Issue 4
Evidence of Solid-Solution Reaction upon Lithium Insertion into Cryptomelane K 0.25 Mn 2 O 4 Material
journal, February 2014
- Pang, Wei Kong; Peterson, Vanessa K.; Sharma, Neeraj
- The Journal of Physical Chemistry C, Vol. 118, Issue 8
The influence of large cations on the electrochemical properties of tunnel-structured metal oxides
journal, November 2016
- Yuan, Yifei; Zhan, Chun; He, Kun
- Nature Communications, Vol. 7, Issue 1
Structural and electrochemical studies of α-manganese dioxide (α-MnO2)
journal, October 1997
- Johnson, C. S.; Dees, D. W.; Mansuetto, M. F.
- Journal of Power Sources, Vol. 68, Issue 2
Asynchronous Crystal Cell Expansion during Lithiation of K + -Stabilized α-MnO 2
journal, April 2015
- Yuan, Yifei; Nie, Anmin; Odegard, Gregory M.
- Nano Letters, Vol. 15, Issue 5
Tracking lithium transport and electrochemical reactions in nanoparticles
journal, January 2012
- Wang, Feng; Yu, Hui-Chia; Chen, Min-Hua
- Nature Communications, Vol. 3, Issue 1
Visualization of lithium-ion transport and phase evolution within and between manganese oxide nanorods
journal, May 2017
- Xu, Feng; Wu, Lijun; Meng, Qingping
- Nature Communications, Vol. 8, Issue 1
Deliberately Designed Atomic-Level Silver-Containing Interface Results in Improved Rate Capability and Utilization of Silver Hollandite for Lithium-Ion Storage
journal, December 2017
- Smith, Paul F.; Brady, Alexander B.; Lee, Seung-Yong
- ACS Applied Materials & Interfaces, Vol. 10, Issue 1
Capture Lithium in αMnO 2 : Insights from First Principles
journal, October 2012
- Ling, Chen; Mizuno, Fuminori
- Chemistry of Materials, Vol. 24, Issue 20
Electrochemistry of Hollandite α-MnO 2 : Li-Ion and Na-Ion Insertion and Li 2 O Incorporation
journal, June 2013
- Tompsett, David A.; Islam, M. Saiful
- Chemistry of Materials, Vol. 25, Issue 12
Understanding the Electrochemical Mechanism of K-αMnO 2 for Magnesium Battery Cathodes
journal, May 2014
- Arthur, Timothy S.; Zhang, Ruigang; Ling, Chen
- ACS Applied Materials & Interfaces, Vol. 6, Issue 10
α-MnO2 as a cathode material for rechargeable Mg batteries
journal, September 2012
- Zhang, Ruigang; Yu, Xiqian; Nam, Kyung-Wan
- Electrochemistry Communications, Vol. 23
Potassium-Based α-Manganese Dioxide Nanofiber Binder-Free Self-Supporting Electrodes: A Design Strategy for High Energy Density Batteries
journal, May 2016
- Poyraz, Altug S.; Huang, Jianping; Wu, Lijun
- Energy Technology, Vol. 4, Issue 11
Works referencing / citing this record:
Solid electrolyte interphases for high-energy aqueous aluminum electrochemical cells
journal, November 2018
- Zhao, Qing; Zachman, Michael J.; Al Sadat, Wajdi I.
- Science Advances, Vol. 4, Issue 11
Capacity Retention for (De)lithiation of Silver Containing α-MnO 2 : Impact of Structural Distortion and Transition Metal Dissolution
journal, January 2018
- Huang, Jianping; Housel, Lisa M.; Quilty, Calvin D.
- Journal of The Electrochemical Society, Vol. 165, Issue 11
Silver-Containing α-MnO 2 Nanorods: Electrochemistry in Rechargeable Aqueous Zn-MnO 2 Batteries
journal, January 2019
- Wang, Lei; Wu, Qiyuan; Abraham, Alyson
- Journal of The Electrochemical Society, Vol. 166, Issue 15
K + pre-intercalated manganese dioxide with enhanced Zn 2+ diffusion for high rate and durable aqueous zinc-ion batteries
journal, January 2019
- Liu, Guoxue; Huang, Huawen; Bi, Ran
- Journal of Materials Chemistry A, Vol. 7, Issue 36
Tunable nanomechanical performance regimes in ceramic nanowires
journal, September 2019
- Maksud, Mahjabin; Barua, Mathius; Shikder, Md Ruhul Amin
- Nanotechnology, Vol. 30, Issue 47
Incorporating Pb2+ Templates into the Crystalline Structure of MnO2 Catalyst Supported on Monolith: Applications in H2O2 Decomposition
journal, September 2019
- Hasanpour, Fatemeh; Saien, Javad
- ACS Omega, Vol. 4, Issue 15
Figures / Tables found in this record:

 Search WorldCat to find libraries that may hold this journal
Search WorldCat to find libraries that may hold this journal