Preliminary neutron diffraction analysis of challenging human manganese superoxide dismutase crystals
Abstract
Superoxide dismutases (SODs) are enzymes that protect against oxidative stress by dismutation of superoxide into oxygen and hydrogen peroxide through cyclic reduction and oxidation of the active-site metal. The complete enzymatic mechanisms of SODs are unknown since data on the positions of hydrogen are limited. Here, methods are presented for large crystal growth and neutron data collection of human manganese SOD (MnSOD) using perdeuteration and the MaNDi beamline at Oak Ridge National Laboratory. The crystal from which the human MnSOD data set was obtained is the crystal with the largest unit-cell edge (240 Å) from which data have been collected via neutron diffraction to sufficient resolution (2.30 Å) where hydrogen positions can be observed.
- Authors:
- Publication Date:
- Research Org.:
- Oak Ridge National Laboratory (ORNL), Oak Ridge, TN (United States)
- Sponsoring Org.:
- USDOE Office of Science (SC), Basic Energy Sciences (BES); National Aeronautics and Space Administration (NASA); National Institutes of Health (NIH)
- OSTI Identifier:
- 1437709
- Alternate Identifier(s):
- OSTI ID: 1407982
- Grant/Contract Number:
- AC05-00OR22725; 44-0307-1021-201; P30CA036727; 5P20RR016469
- Resource Type:
- Published Article
- Journal Name:
- Acta Crystallographica. Section F, Structural Biology Communications
- Additional Journal Information:
- Journal Name: Acta Crystallographica. Section F, Structural Biology Communications Journal Volume: 73 Journal Issue: 4; Journal ID: ISSN 2053-230X
- Publisher:
- International Union of Crystallography (IUCr)
- Country of Publication:
- United Kingdom
- Language:
- English
- Subject:
- 37 INORGANIC, ORGANIC, PHYSICAL, AND ANALYTICAL CHEMISTRY; 60 APPLIED LIFE SCIENCES; manganese superoxide dismutase; neutron diffraction; perdeuteration; human; large unit cell
Citation Formats
Azadmanesh, Jahaun, Trickel, Scott R., Weiss, Kevin L., Coates, Leighton, and Borgstahl, Gloria E. O. Preliminary neutron diffraction analysis of challenging human manganese superoxide dismutase crystals. United Kingdom: N. p., 2017.
Web. doi:10.1107/S2053230X17003508.
Azadmanesh, Jahaun, Trickel, Scott R., Weiss, Kevin L., Coates, Leighton, & Borgstahl, Gloria E. O. Preliminary neutron diffraction analysis of challenging human manganese superoxide dismutase crystals. United Kingdom. https://doi.org/10.1107/S2053230X17003508
Azadmanesh, Jahaun, Trickel, Scott R., Weiss, Kevin L., Coates, Leighton, and Borgstahl, Gloria E. O. Wed .
"Preliminary neutron diffraction analysis of challenging human manganese superoxide dismutase crystals". United Kingdom. https://doi.org/10.1107/S2053230X17003508.
@article{osti_1437709,
title = {Preliminary neutron diffraction analysis of challenging human manganese superoxide dismutase crystals},
author = {Azadmanesh, Jahaun and Trickel, Scott R. and Weiss, Kevin L. and Coates, Leighton and Borgstahl, Gloria E. O.},
abstractNote = {Superoxide dismutases (SODs) are enzymes that protect against oxidative stress by dismutation of superoxide into oxygen and hydrogen peroxide through cyclic reduction and oxidation of the active-site metal. The complete enzymatic mechanisms of SODs are unknown since data on the positions of hydrogen are limited. Here, methods are presented for large crystal growth and neutron data collection of human manganese SOD (MnSOD) using perdeuteration and the MaNDi beamline at Oak Ridge National Laboratory. The crystal from which the human MnSOD data set was obtained is the crystal with the largest unit-cell edge (240 Å) from which data have been collected via neutron diffraction to sufficient resolution (2.30 Å) where hydrogen positions can be observed.},
doi = {10.1107/S2053230X17003508},
journal = {Acta Crystallographica. Section F, Structural Biology Communications},
number = 4,
volume = 73,
place = {United Kingdom},
year = {Wed Mar 29 00:00:00 EDT 2017},
month = {Wed Mar 29 00:00:00 EDT 2017}
}
https://doi.org/10.1107/S2053230X17003508
Web of Science
Figures / Tables:
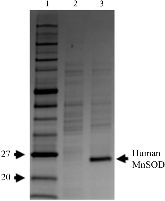 Figure 1: Fermentor growth of perdeuterated human MnSOD. SDS–PAGE of whole-cell lysate from cells grown in perdeuterated media immediately before and 13 h after induction. Monomeric human MnSOD has a molecular weight of 22 kDa. Samples were normalized to equivalent optical densities. Lane 1, molecular-mass markers (labelled in kDa). Lanemore »
Figure 1: Fermentor growth of perdeuterated human MnSOD. SDS–PAGE of whole-cell lysate from cells grown in perdeuterated media immediately before and 13 h after induction. Monomeric human MnSOD has a molecular weight of 22 kDa. Samples were normalized to equivalent optical densities. Lane 1, molecular-mass markers (labelled in kDa). Lanemore »
Works referenced in this record:
Construction of an Escherichia coli K-12 strain deleted for manganese and iron superoxide dismutase genes and its use in cloning the iron superoxide dismutase gene of Legionella pneumophila
journal, April 1992
- Steinman, HowardM.
- MGG Molecular & General Genetics, Vol. 232, Issue 3
Role of Hydrogen Bonding in the Active Site of Human Manganese Superoxide Dismutase † , ‡
journal, June 2004
- Greenleaf, William B.; Perry, J. Jefferson P.; Hearn, Amy S.
- Biochemistry, Vol. 43, Issue 22
Interrupting the Hydrogen Bond Network at the Active Site of Human Manganese Superoxide Dismutase
journal, September 1999
- Ramilo, Cecilia A.; Leveque, Vincent; Guan, Yue
- Journal of Biological Chemistry, Vol. 274, Issue 39
LAUEGEN version 6.0 and INTLDM
journal, June 1998
- Campbell, J. W.; Hao, Q.; Harding, M. M.
- Journal of Applied Crystallography, Vol. 31, Issue 3
Mantid—Data analysis and visualization package for neutron scattering and SR experiments
journal, November 2014
- Arnold, O.; Bilheux, J. C.; Borreguero, J. M.
- Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment, Vol. 764
Protein structures by spallation neutron crystallography
journal, April 2008
- Langan, Paul; Fisher, Zoë; Kovalevsky, Andrii
- Journal of Synchrotron Radiation, Vol. 15, Issue 3
Rapid sampling, cell inactivation and evaluation of low extracellular glucose concentrations during fed-batch cultivation
journal, August 1996
- Larsson, Gen; Törnkvist, Mari
- Journal of Biotechnology, Vol. 49, Issue 1-3
Overview of the CCP 4 suite and current developments
journal, March 2011
- Winn, Martyn D.; Ballard, Charles C.; Cowtan, Kevin D.
- Acta Crystallographica Section D Biological Crystallography, Vol. 67, Issue 4
The Macromolecular Neutron Diffractometer MaNDi at the Spallation Neutron Source
journal, July 2015
- Coates, Leighton; Cuneo, Matthew J.; Frost, Matthew J.
- Journal of Applied Crystallography, Vol. 48, Issue 4
Deuterium Labeling for Neutron Structure-Function-Dynamics Analysis
book, January 2009
- Meilleur, Flora; Weiss, Kevin L.; Myles, Dean A. A.
- Micro and Nano Technologies in Bioanalysis
Theoretical Studies of Manganese and Iron Superoxide Dismutases: Superoxide Binding and Superoxide Oxidation
journal, December 2005
- Abreu, Isabel A.; Rodriguez, José A.; Cabelli, Diane E.
- The Journal of Physical Chemistry B, Vol. 109, Issue 51
Developing master keys to brain pathology, cancer and aging from the structural biology of proteins controlling reactive oxygen species and DNA repair
journal, April 2007
- Perry, J. J. P.; Fan, L.; Tainer, J. A.
- Neuroscience, Vol. 145, Issue 4
Structure-function in Escherichia coli iron superoxide dismutase: Comparisons with the manganese enzyme from Thermus thermophilus
journal, February 1995
- Lah, Myoung S.; Dixon, Melinda M.; Pattridge, Katherine A.
- Biochemistry, Vol. 34, Issue 5
The macromolecular neutron diffractometer (MaNDi) at the Spallation Neutron Source, Oak Ridge: enhanced optics design, high-resolution neutron detectors and simulated diffraction
journal, April 2010
- Coates, L.; Stoica, A. D.; Hoffmann, C.
- Journal of Applied Crystallography, Vol. 43, Issue 3
Mitochondrial proton and electron leaks
journal, June 2010
- Jastroch, Martin; Divakaruni, Ajit S.; Mookerjee, Shona
- Essays in Biochemistry, Vol. 47
Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase
journal, December 1995
- Li, Yibing; Huang, Ting-Ting; Carlson, Elaine J.
- Nature Genetics, Vol. 11, Issue 4
When X-rays modify the protein structure: radiation damage at work
journal, April 2005
- Carugo, Oliviero; Carugo, Kristina Djinović
- Trends in Biochemical Sciences, Vol. 30, Issue 4
Modulation of MnSOD in Cancer: Epidemiological and Experimental Evidences
journal, June 2010
- Kim, Ae-Kyong
- Toxicological Research, Vol. 26, Issue 2
Catalytic and Structural Effects of Amino Acid Substitution at Histidine 30 in Human Manganese Superoxide Dismutase: Insertion of Valine Cγ into the Substrate Access Channel †
journal, March 2003
- Hearn, Amy S.; Stroupe, M. Elizabeth; Cabelli, Diane E.
- Biochemistry, Vol. 42, Issue 10
Redox Properties of Human Manganese Superoxide Dismutase and Active-Site Mutants †
journal, September 2001
- Lévêque, Vincent J. -P.; Vance, Carrie K.; Nick, Harry S.
- Biochemistry, Vol. 40, Issue 35
Multiple Replacements of Glutamine 143 in Human Manganese Superoxide Dismutase: Effects on Structure, Stability, and Catalysis †
journal, June 2000
- Lévêque, Vincent J. -P.; Stroupe, M. Elizabeth; Lepock, James R.
- Biochemistry, Vol. 39, Issue 24
XDS
journal, January 2010
- Kabsch, Wolfgang
- Acta Crystallographica Section D Biological Crystallography, Vol. 66, Issue 2
Proton-coupled electron transfer in Fe-superoxide dismutase and Mn-superoxide dismutase
journal, January 2003
- Miller, Anne-Frances; Padmakumar, K.; Sorkin, David L.
- Journal of Inorganic Biochemistry, Vol. 93, Issue 1-2
Superoxide dismutases—a review of the metal-associated mechanistic variations
journal, February 2010
- Abreu, Isabel A.; Cabelli, Diane E.
- Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics, Vol. 1804, Issue 2
Structural and Functional Aspects of Metal Sites in Biology
journal, January 1996
- Holm, Richard H.; Kennepohl, Pierre; Solomon, Edward I.
- Chemical Reviews, Vol. 96, Issue 7
Neutron macromolecular crystallography
journal, June 2009
- Blakeley, M. P.
- Crystallography Reviews, Vol. 15, Issue 3
Figures / Tables found in this record:

 Search WorldCat to find libraries that may hold this journal
Search WorldCat to find libraries that may hold this journal