Redox-Mediated Stabilization in Zinc Molybdenum Nitrides
Abstract
We report on the theoretical prediction and experimental realization of new ternary zinc molybdenum nitride compounds. We used theory to identify previously unknown ternary compounds in the Zn-Mo-N systems, Zn3MoN4 and ZnMoN2, and to analyze their bonding environment. Experiments show that Zn-Mo-N alloys can form in broad composition range from Zn3MoN4 to ZnMoN2 in the wurtzite-derived structure, accommodating very large off-stoichiometry. Interestingly, the measured wurtzite-derived structure of the alloys is metastable for the ZnMoN2 stoichiometry, in contrast to the Zn3MoN4 stoichiometry, where ordered wurtzite is predicted to be the ground state. The formation of Zn3MoN4-ZnMoN2 alloy with wurtzite-derived crystal structure is enabled by the concomitant ability of Mo to change oxidation state from +VI in Zn3MoN4 to +IV in ZnMoN2, and the capability of Zn to contribute to the bonding states of both compounds, an effect that we define as "redox-mediated stabilization". The stabilization of Mo in both the +VI and +IV oxidation states is due to the intermediate electronegativity of Zn, which enables significant polar covalent bonding in both Zn3MoN4 and ZnMoN2 compounds. The smooth change in the Mo oxidation state between Zn3MoN4 and ZnMoN2 stoichiometries leads to a continuous change in optoelectronic properties - from resistive and semitransparentmore »
- Authors:
-
- National Renewable Energy Lab. (NREL), Golden, CO (United States)
- Univ. of Colorado, Boulder, CO (United States)
- Colorado School of Mines, Golden, CO (United States)
- Lawrence Berkeley National Lab. (LBNL), Berkeley, CA (United States)
- National Renewable Energy Lab. (NREL), Golden, CO (United States); Univ. of Colorado, Boulder, CO (United States)
- Lawrence Berkeley National Lab. (LBNL), Berkeley, CA (United States); Univ. of California, Berkeley, CA (United States)
- Publication Date:
- Research Org.:
- National Renewable Energy Lab. (NREL), Golden, CO (United States); Energy Frontier Research Centers (EFRC) (United States). Center for Next Generation of Materials by Design: Incorporating Metastability (CNGMD); Lawrence Berkeley National Lab. (LBNL), Berkeley, CA (United States)
- Sponsoring Org.:
- USDOE Office of Science (SC), Basic Energy Sciences (BES)
- OSTI Identifier:
- 1429974
- Alternate Identifier(s):
- OSTI ID: 1530347
- Report Number(s):
- NREL/JA-5K00-70614
Journal ID: ISSN 0002-7863; TRN: US1802486
- Grant/Contract Number:
- AC36-08GO28308; AC02-05CH11231
- Resource Type:
- Accepted Manuscript
- Journal Name:
- Journal of the American Chemical Society
- Additional Journal Information:
- Journal Volume: 140; Journal Issue: 12; Journal ID: ISSN 0002-7863
- Publisher:
- American Chemical Society (ACS)
- Country of Publication:
- United States
- Language:
- English
- Subject:
- 36 MATERIALS SCIENCE; 71 CLASSICAL AND QUANTUM MECHANICS, GENERAL PHYSICS; zinc molybdenum nitrides; ternary compounds; composition
Citation Formats
Arca, Elisabetta, Lany, Stephan, Perkins, John D., Bartel, Christopher, Mangum, John, Sun, Wenhao, Holder, Aaron, Ceder, Gerbrand, Gorman, Brian, Teeter, Glenn, Tumas, William, and Zakutayev, Andriy. Redox-Mediated Stabilization in Zinc Molybdenum Nitrides. United States: N. p., 2018.
Web. doi:10.1021/jacs.7b12861.
Arca, Elisabetta, Lany, Stephan, Perkins, John D., Bartel, Christopher, Mangum, John, Sun, Wenhao, Holder, Aaron, Ceder, Gerbrand, Gorman, Brian, Teeter, Glenn, Tumas, William, & Zakutayev, Andriy. Redox-Mediated Stabilization in Zinc Molybdenum Nitrides. United States. https://doi.org/10.1021/jacs.7b12861
Arca, Elisabetta, Lany, Stephan, Perkins, John D., Bartel, Christopher, Mangum, John, Sun, Wenhao, Holder, Aaron, Ceder, Gerbrand, Gorman, Brian, Teeter, Glenn, Tumas, William, and Zakutayev, Andriy. Thu .
"Redox-Mediated Stabilization in Zinc Molybdenum Nitrides". United States. https://doi.org/10.1021/jacs.7b12861. https://www.osti.gov/servlets/purl/1429974.
@article{osti_1429974,
title = {Redox-Mediated Stabilization in Zinc Molybdenum Nitrides},
author = {Arca, Elisabetta and Lany, Stephan and Perkins, John D. and Bartel, Christopher and Mangum, John and Sun, Wenhao and Holder, Aaron and Ceder, Gerbrand and Gorman, Brian and Teeter, Glenn and Tumas, William and Zakutayev, Andriy},
abstractNote = {We report on the theoretical prediction and experimental realization of new ternary zinc molybdenum nitride compounds. We used theory to identify previously unknown ternary compounds in the Zn-Mo-N systems, Zn3MoN4 and ZnMoN2, and to analyze their bonding environment. Experiments show that Zn-Mo-N alloys can form in broad composition range from Zn3MoN4 to ZnMoN2 in the wurtzite-derived structure, accommodating very large off-stoichiometry. Interestingly, the measured wurtzite-derived structure of the alloys is metastable for the ZnMoN2 stoichiometry, in contrast to the Zn3MoN4 stoichiometry, where ordered wurtzite is predicted to be the ground state. The formation of Zn3MoN4-ZnMoN2 alloy with wurtzite-derived crystal structure is enabled by the concomitant ability of Mo to change oxidation state from +VI in Zn3MoN4 to +IV in ZnMoN2, and the capability of Zn to contribute to the bonding states of both compounds, an effect that we define as "redox-mediated stabilization". The stabilization of Mo in both the +VI and +IV oxidation states is due to the intermediate electronegativity of Zn, which enables significant polar covalent bonding in both Zn3MoN4 and ZnMoN2 compounds. The smooth change in the Mo oxidation state between Zn3MoN4 and ZnMoN2 stoichiometries leads to a continuous change in optoelectronic properties - from resistive and semitransparent Zn3MoN4 to conductive and absorptive ZnMoN2. The reported redox-mediated stabilization in zinc molybdenum nitrides suggests there might be many undiscovered ternary compounds with one metal having an intermediate electronegativity, enabling significant covalent bonding, and another metal capable of accommodating multiple oxidation states, enabling stoichiometric flexibility.},
doi = {10.1021/jacs.7b12861},
journal = {Journal of the American Chemical Society},
number = 12,
volume = 140,
place = {United States},
year = {Thu Mar 01 00:00:00 EST 2018},
month = {Thu Mar 01 00:00:00 EST 2018}
}
Web of Science
Figures / Tables:
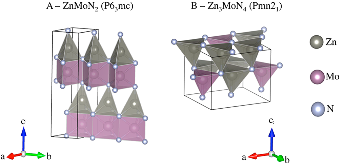 Figure 1: Predicted crystal structures of the (A) ZnMoN2 with Mo in 6-fold coordination and (B) Zn3MoN4 compounds with Mo in 4-fold coordination. For Zn, 4-fold coordination is observed in both structures. The Mo coordination polyhedrons are magenta, and the Zn coordination polyhedrons are gray.
Figure 1: Predicted crystal structures of the (A) ZnMoN2 with Mo in 6-fold coordination and (B) Zn3MoN4 compounds with Mo in 4-fold coordination. For Zn, 4-fold coordination is observed in both structures. The Mo coordination polyhedrons are magenta, and the Zn coordination polyhedrons are gray.
Works referenced in this record:
Design of nitride semiconductors for solar energy conversion
journal, January 2016
- Zakutayev, Andriy
- Journal of Materials Chemistry A, Vol. 4, Issue 18
The high-throughput highway to computational materials design
journal, February 2013
- Curtarolo, Stefano; Hart, Gus L. W.; Nardelli, Marco Buongiorno
- Nature Materials, Vol. 12, Issue 3
The thermodynamic scale of inorganic crystalline metastability
journal, November 2016
- Sun, Wenhao; Dacek, Stephen T.; Ong, Shyue Ping
- Science Advances, Vol. 2, Issue 11
A Review of Epitaxial Metal-Nitride Films by Polymer-Assisted Deposition
journal, April 2010
- Luo, Hongmei; Wang, Haiyan; Zou, Guifu
- Transactions on Electrical and Electronic Materials, Vol. 11, Issue 2
Formation of transition-metal nitrides from the reactions of lithium amides and anhydrous transition-metal chlorides
journal, January 1995
- Parkin, Ivan P.; Rowley, Adrian T.
- Journal of Materials Chemistry, Vol. 5, Issue 6
High-pressure chemistry of nitride-based materials
journal, January 2006
- Horvath-Bordon, Elisabeta; Riedel, Ralf; Zerr, Andreas
- Chemical Society Reviews, Vol. 35, Issue 10
Synthesis of a mixed-valent tin nitride and considerations of its possible crystal structures
journal, April 2016
- Caskey, Christopher M.; Holder, Aaron; Shulda, Sarah
- The Journal of Chemical Physics, Vol. 144, Issue 14
Thermodynamic Routes to Novel Metastable Nitrogen-Rich Nitrides
journal, August 2017
- Sun, Wenhao; Holder, Aaron; Orvañanos, Bernardo
- Chemistry of Materials, Vol. 29, Issue 16
Exploring the PbS–Bi 2 S 3 Series for Next Generation Energy Conversion Materials
journal, May 2017
- Savory, Christopher N.; Ganose, Alex M.; Scanlon, David O.
- Chemistry of Materials, Vol. 29, Issue 12
From the computer to the laboratory: materials discovery and design using first-principles calculations
journal, May 2012
- Hautier, Geoffroy; Jain, Anubhav; Ong, Shyue Ping
- Journal of Materials Science, Vol. 47, Issue 21
Discovery of earth-abundant nitride semiconductors by computational screening and high-pressure synthesis
journal, June 2016
- Hinuma, Yoyo; Hatakeyama, Taisuke; Kumagai, Yu
- Nature Communications, Vol. 7, Issue 1
Synthesis and Structure of Two New Ternary Nitrides: FeWN 2 and MnMoN 2
journal, January 1996
- Bem, David S.; Lampe-Önnerud, Christina M.; Olsen, Hans P.
- Inorganic Chemistry, Vol. 35, Issue 3
Prediction of Stable Nitride Perovskites
journal, August 2015
- Sarmiento-Pérez, Rafael; Cerqueira, Tiago F. T.; Körbel, Sabine
- Chemistry of Materials, Vol. 27, Issue 17
Synthesis and characterization of a binary noble metal nitride
journal, April 2004
- Gregoryanz, Eugene; Sanloup, Chrystele; Somayazulu, M.
- Nature Materials, Vol. 3, Issue 5
High-Pressure Synthesis and Characterization of Incompressible Titanium Pernitride
journal, February 2016
- Bhadram, Venkata S.; Kim, Duck Young; Strobel, Timothy A.
- Chemistry of Materials, Vol. 28, Issue 6
Synthesis, characterisation and hydrogenation performance of ternary nitride catalysts
journal, November 2014
- Perret, Noémie; Alexander, Anne-Marie; Hunter, Stuart M.
- Applied Catalysis A: General, Vol. 488
Mixed Close-Packed Cobalt Molybdenum Nitrides as Non-noble Metal Electrocatalysts for the Hydrogen Evolution Reaction
journal, December 2013
- Cao, Bingfei; Veith, Gabriel M.; Neuefeind, Joerg C.
- Journal of the American Chemical Society, Vol. 135, Issue 51
Metal Nitrides Grown from Ca/Li Flux: Ca 6 Te 3 N 2 and New Nitridoferrate(I) Ca 6 (Li x Fe 1– x )Te 2 N 3
journal, August 2016
- Dickman, Matthew J.; Latturner, Susan E.
- Journal of the American Chemical Society, Vol. 138, Issue 33
Infrared Plasmonics with Conductive Ternary Nitrides
journal, March 2017
- Metaxa, C.; Kassavetis, S.; Pierson, J. F.
- ACS Applied Materials & Interfaces, Vol. 9, Issue 12
Two-Dimensional Layered Complex Nitrides as a New Class of Thermoelectric Materials
journal, April 2014
- Ohkubo, Isao; Mori, Takao
- Chemistry of Materials, Vol. 26, Issue 8
Group V and VI Alkali Nitridometalates: A Growing Class of Compounds with Structures Related to Silicate Chemistry
journal, January 1996
- Niewa, Rainer; Jacobs, Herbert
- Chemical Reviews, Vol. 96, Issue 6
Crystal structure of tristrontium [tetranitridomolybdate(VI)], Sr3[MoN4]
journal, March 2000
- Höhn, P.; Kniep, R.
- Zeitschrift für Kristallographie - New Crystal Structures, Vol. 215, Issue 3
Chemistry of interstitial molybdenum ternary nitrides MnMo3N (M=Fe, Co, n=3; M=Ni, n=2)
journal, January 1998
- Alconchel, Silvia; Sapiña, Fernando; Beltrán, Daniel
- Journal of Materials Chemistry, Vol. 8, Issue 8
Molybdenum enzymes, cofactors, and systems: The chemical uniqueness of molybdenum
journal, November 1985
- Burgmayer, S. J. N.; Stiefel, E. I.
- Journal of Chemical Education, Vol. 62, Issue 11
Light-driven dinitrogen reduction catalyzed by a CdS:nitrogenase MoFe protein biohybrid
journal, April 2016
- Brown, K. A.; Harris, D. F.; Wilker, M. B.
- Science, Vol. 352, Issue 6284
Oxidation-State-Dependent Binding Properties of the Active Site in a Mo-Containing Formate Dehydrogenase
journal, July 2017
- Robinson, William E.; Bassegoda, Arnau; Reisner, Erwin
- Journal of the American Chemical Society, Vol. 139, Issue 29
3D Porous Hierarchical Nickel-Molybdenum Nitrides Synthesized by RF Plasma as Highly Active and Stable Hydrogen-Evolution-Reaction Electrocatalysts
journal, April 2016
- Zhang, Yongqi; Ouyang, Bo; Xu, Jing
- Advanced Energy Materials, Vol. 6, Issue 11
Monte Carlo simulations of disorder in and the effects on the electronic structure
journal, August 2017
- Lany, Stephan; Fioretti, Angela N.; Zawadzki, Paweł P.
- Physical Review Materials, Vol. 1, Issue 3
Synthesis and structure of new mixed alkaline-earth nitridomolybdates and nitridotungstates, (Ba,Ca)3[MN4] (M = Mo, W)Dedicated to Dr Marten G. Barker in memoriam.
journal, February 2003
- Baker, Charles F.; Barker, Marten G.; Blake, Alexander J.
- Dalton Transactions, Issue 6
Sr10[Mo2N6][MoN4]2 and ?-Sr3MoN4
journal, November 2004
- Bailey, Mark S.; McGuire, Michael A.; DiSalvo, Francis J.
- Zeitschrift f�r anorganische und allgemeine Chemie, Vol. 630, Issue 13-14
Salt-Templated Synthesis of 2D Metallic MoN and Other Nitrides
journal, February 2017
- Xiao, Xu; Yu, Huimin; Jin, Huanyu
- ACS Nano, Vol. 11, Issue 2
Theoretical and Experimental Study of the Electronic Structures of MoO 3 and MoO 2
journal, March 2010
- Scanlon, David O.; Watson, Graeme W.; Payne, D. J.
- The Journal of Physical Chemistry C, Vol. 114, Issue 10
Bandgap engineering of ZnSnP 2 for high-efficiency solar cells
journal, June 2012
- Scanlon, David O.; Walsh, Aron
- Applied Physics Letters, Vol. 100, Issue 25
Band Gap Dependence on Cation Disorder in ZnSnN 2 Solar Absorber
journal, October 2015
- Veal, Tim D.; Feldberg, Nathaniel; Quackenbush, Nicholas F.
- Advanced Energy Materials, Vol. 5, Issue 24
Charge-neutral disorder and polytypes in heterovalent wurtzite-based ternary semiconductors: The importance of the octet rule
journal, May 2015
- Quayle, Paul C.; Blanton, Eric W.; Punya, Atchara
- Physical Review B, Vol. 91, Issue 20
Design of Metastable Tin Titanium Nitride Semiconductor Alloys
journal, July 2017
- Bikowski, Andre; Siol, Sebastian; Gu, Jing
- Chemistry of Materials, Vol. 29, Issue 15
Novel phase diagram behavior and materials design in heterostructural semiconductor alloys
journal, June 2017
- Holder, Aaron M.; Siol, Sebastian; Ndione, Paul F.
- Science Advances, Vol. 3, Issue 6
Control of Doping in Cu 2 SnS 3 through Defects and Alloying
journal, August 2014
- Baranowski, Lauryn L.; Zawadzki, Pawel; Christensen, Steven
- Chemistry of Materials, Vol. 26, Issue 17
Li-Doped Cr 2 MnO 4 : A New p-Type Transparent Conducting Oxide by Computational Materials Design
journal, May 2013
- Peng, Haowei; Zakutayev, Andriy; Lany, Stephan
- Advanced Functional Materials, Vol. 23, Issue 42
Development and application of an instrument for spatially resolved Seebeck coefficient measurements
journal, May 2013
- Zakutayev, Andriy; Luciano, Frank J.; Bollinger, Vincent P.
- Review of Scientific Instruments, Vol. 84, Issue 5
Finding Nature’s Missing Ternary Oxide Compounds Using Machine Learning and Density Functional Theory
journal, June 2010
- Hautier, Geoffroy; Fischer, Christopher C.; Jain, Anubhav
- Chemistry of Materials, Vol. 22, Issue 12
Exploring the Real Ground-State Structures of Molybdenum Dinitride
journal, May 2016
- Yu, Shuyin; Huang, Bowen; Jia, Xiaojing
- The Journal of Physical Chemistry C, Vol. 120, Issue 20
FINDSYM : program for identifying the space-group symmetry of a crystal
journal, January 2005
- Stokes, Harold T.; Hatch, Dorian M.
- Journal of Applied Crystallography, Vol. 38, Issue 1
Implementation and performance of the frequency-dependent method within the PAW framework
journal, July 2006
- Shishkin, M.; Kresse, G.
- Physical Review B, Vol. 74, Issue 3
Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA+U study
journal, January 1998
- Dudarev, S. L.; Botton, G. A.; Savrasov, S. Y.
- Physical Review B, Vol. 57, Issue 3, p. 1505-1509
Van der Waals density functionals applied to solids
journal, May 2011
- Klimeš, Jiří; Bowler, David R.; Michaelides, Angelos
- Physical Review B, Vol. 83, Issue 19
Correcting density functional theory for accurate predictions of compound enthalpies of formation: Fitted elemental-phase reference energies
journal, March 2012
- Stevanović, Vladan; Lany, Stephan; Zhang, Xiuwen
- Physical Review B, Vol. 85, Issue 11
LOBSTER: A tool to extract chemical bonding from plane-wave based DFT: Tool to Extract Chemical Bonding
journal, February 2016
- Maintz, Stefan; Deringer, Volker L.; Tchougréeff, Andrei L.
- Journal of Computational Chemistry, Vol. 37, Issue 11
Works referencing / citing this record:
A map of the inorganic ternary metal nitrides
journal, June 2019
- Sun, Wenhao; Bartel, Christopher J.; Arca, Elisabetta
- Nature Materials, Vol. 18, Issue 7
Blue-green emission from epitaxial yet cation-disordered
journal, May 2019
- Melamed, Celeste L.; Tellekamp, M. Brooks; Mangum, John S.
- Physical Review Materials, Vol. 3, Issue 5
Physical descriptor for the Gibbs energy of inorganic crystalline solids and temperature-dependent materials chemistry
journal, October 2018
- Bartel, Christopher J.; Millican, Samantha L.; Deml, Ann M.
- Nature Communications, Vol. 9, Issue 1
Ternary nitride semiconductors in the rocksalt crystal structure
journal, July 2019
- Bauers, Sage R.; Holder, Aaron; Sun, Wenhao
- Proceedings of the National Academy of Sciences, Vol. 116, Issue 30
A Map of the Inorganic Ternary Metal Nitrides
text, January 2018
- Sun, Wenhao; Bartel, Christopher; Arca, Elisabetta
- arXiv

 Search WorldCat to find libraries that may hold this journal
Search WorldCat to find libraries that may hold this journal