Effect of molecular shape on rotation under severe confinement
Abstract
Orientational structure and dynamics of molecules is known to be affected by confinement in space comparable in size to the molecule itself. ZSM-5 with porous channels of ≈0.55 nm is such a porous medium, which offers a strict spatial confinement on low molecular weight hydrocarbons. An important factor that determines these properties is the shape of the confined molecules. In this work, we employed molecular dynamics simulation to study the orientational structure and dynamics of four molecules that differ in shape but have similar kinetic diameters and moments of inertia, confined in ZSM-5. The effect of molecular shape on the orientational structure and dynamics of propane, acetonitrile, acetaldehyde and acetone in ZSM-5 is studied by means of probing the differences in the orientational distribution of molecules in the ZSM-5 channels, and extracting time scales of the decay of correlation functions related to rotational motion. Orientational correlation functions of all the four molecules exhibit two regimes of rotational motion. While the short time regime represents free rotation of the molecules before they collide with the pore walls, the long time orientational jumps driven by inter-channel migrations give rise to a very slow varying second regime. Of the molecules studied, orientational structuremore »
- Authors:
-
- Oak Ridge National Lab. (ORNL), Oak Ridge, TN (United States). Chemical and Engineering Materials Division
- Oak Ridge National Lab. (ORNL), Oak Ridge, TN (United States). Computational Science and Engineering Division
- University of Tennessee/Oak Ridge National Laboratory, Oak Ridge, TN (United States). Center for Molecular Biophysics
- The Ohio State University, Columbus, OH (United States). School of Earth Sciences
- Publication Date:
- Research Org.:
- Oak Ridge National Lab. (ORNL), Oak Ridge, TN (United States)
- Sponsoring Org.:
- USDOE Office of Science (SC), Basic Energy Sciences (BES). Chemical Sciences, Geosciences, and Biosciences Division
- OSTI Identifier:
- 1422992
- Alternate Identifier(s):
- OSTI ID: 1573263
- Grant/Contract Number:
- AC05-00OR22725; SC0006878
- Resource Type:
- Accepted Manuscript
- Journal Name:
- Chemical Engineering Science
- Additional Journal Information:
- Journal Volume: 180; Journal Issue: C; Journal ID: ISSN 0009-2509
- Publisher:
- Elsevier
- Country of Publication:
- United States
- Language:
- English
- Subject:
- 37 INORGANIC, ORGANIC, PHYSICAL, AND ANALYTICAL CHEMISTRY; 42 ENGINEERING; ZSM-5; Molecular rotation; Propane; Acetone; Acetaldehyde; Acetonitrile
Citation Formats
Dhiman, Indu, Bhowmik, Debsindhu, Shrestha, Utsab R., Cole, David R., and Gautam, Siddharth. Effect of molecular shape on rotation under severe confinement. United States: N. p., 2018.
Web. doi:10.1016/j.ces.2018.01.027.
Dhiman, Indu, Bhowmik, Debsindhu, Shrestha, Utsab R., Cole, David R., & Gautam, Siddharth. Effect of molecular shape on rotation under severe confinement. United States. https://doi.org/10.1016/j.ces.2018.01.027
Dhiman, Indu, Bhowmik, Debsindhu, Shrestha, Utsab R., Cole, David R., and Gautam, Siddharth. Wed .
"Effect of molecular shape on rotation under severe confinement". United States. https://doi.org/10.1016/j.ces.2018.01.027. https://www.osti.gov/servlets/purl/1422992.
@article{osti_1422992,
title = {Effect of molecular shape on rotation under severe confinement},
author = {Dhiman, Indu and Bhowmik, Debsindhu and Shrestha, Utsab R. and Cole, David R. and Gautam, Siddharth},
abstractNote = {Orientational structure and dynamics of molecules is known to be affected by confinement in space comparable in size to the molecule itself. ZSM-5 with porous channels of ≈0.55 nm is such a porous medium, which offers a strict spatial confinement on low molecular weight hydrocarbons. An important factor that determines these properties is the shape of the confined molecules. In this work, we employed molecular dynamics simulation to study the orientational structure and dynamics of four molecules that differ in shape but have similar kinetic diameters and moments of inertia, confined in ZSM-5. The effect of molecular shape on the orientational structure and dynamics of propane, acetonitrile, acetaldehyde and acetone in ZSM-5 is studied by means of probing the differences in the orientational distribution of molecules in the ZSM-5 channels, and extracting time scales of the decay of correlation functions related to rotational motion. Orientational correlation functions of all the four molecules exhibit two regimes of rotational motion. While the short time regime represents free rotation of the molecules before they collide with the pore walls, the long time orientational jumps driven by inter-channel migrations give rise to a very slow varying second regime. Of the molecules studied, orientational structure and dynamics of propane is found to be least affected by confinement under ZSM-5, whereas charge and shape asymmetry of other molecules makes their interchannel migration-driven rotation slow. The time scales involved in the rotational motion for the molecules studied are compared with similar studies reported in literature. Lastly, this study reveals the important role that molecular shape plays in the behavior of confined molecules.},
doi = {10.1016/j.ces.2018.01.027},
journal = {Chemical Engineering Science},
number = C,
volume = 180,
place = {United States},
year = {Wed Jan 31 00:00:00 EST 2018},
month = {Wed Jan 31 00:00:00 EST 2018}
}
Web of Science
Figures / Tables:
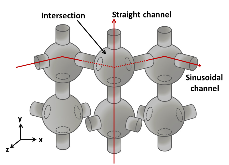 Figure 1: The approximate schematic of ZSM-5 structure in 3D. Different channel structures, straight (vertical line), sinusoidal (zigzag line), and the intersection channels are also depicted.
Figure 1: The approximate schematic of ZSM-5 structure in 3D. Different channel structures, straight (vertical line), sinusoidal (zigzag line), and the intersection channels are also depicted.
Works referenced in this record:
Capillarity-induced filling of carbon nanotubes
journal, January 1993
- Ajayan, P. M.; lijima, Sumio
- Nature, Vol. 361, Issue 6410, p. 333-334
Carbon nanotubes as removable templates for metal oxide nanocomposites and nanostructures
journal, June 1995
- Ajayan, P. M.; Stephan, O.; Redlich, Ph.
- Nature, Vol. 375, Issue 6532
Rotation driven translational diffusion of polyatomic ions in water: A novel mechanism for breakdown of Stokes-Einstein relation
journal, April 2017
- Banerjee, Puja; Yashonath, Subramanian; Bagchi, Biman
- The Journal of Chemical Physics, Vol. 146, Issue 16
Rotational motion of a single water molecule in a buckyball
journal, January 2013
- Farimani, A. Barati; Wu, Yanbin; Aluru, N. R.
- Physical Chemistry Chemical Physics, Vol. 15, Issue 41
Dynamics of supercooled water in confined geometry
journal, January 2000
- Bergman, R.; Swenson, J.
- Nature, Vol. 403, Issue 6767
Study of Translational and Rotational Mobility and Orientational Preference of Ethane in One-Dimensional Channels
journal, August 2002
- Bhide, Shreyas Y.; Yashonath, S.
- The Journal of Physical Chemistry A, Vol. 106, Issue 31
Study of tetrabutylammonium bromide in aqueous solution by neutron scattering
journal, November 2012
- Bhowmik, D.; Malikova, N.; Teixeira, J.
- The European Physical Journal Special Topics, Vol. 213, Issue 1
Aqueous solutions of tetraalkylammonium halides: ion hydration, dynamics and ion–ion interactions in light of steric effects
journal, January 2014
- Bhowmik, Debsindhu; Malikova, Natalie; Mériguet, Guillaume
- Phys. Chem. Chem. Phys., Vol. 16, Issue 26
Properties of liquid acetone in silica pores: Molecular dynamics simulation
journal, April 1996
- Bródka, A.; Zerda, T. W.
- The Journal of Chemical Physics, Vol. 104, Issue 16
Dynamics of liquid acetone: Computer simulation
journal, April 1996
- Bródka, A.; Zerda, T. W.
- The Journal of Chemical Physics, Vol. 104, Issue 16
Structural and dynamical properties of guest molecules confined in mesoporous silica materials revealed by NMR
journal, January 2007
- Buntkowsky, Gerd; Breitzke, Hergen; Adamczyk, Anna
- Physical Chemistry Chemical Physics, Vol. 9, Issue 35
Dynamics of absorbed water in saponite clay: Neutron scattering study
journal, August 2006
- Chakrabarty, Debojit; Gautam, Siddharth; Mitra, S.
- Chemical Physics Letters, Vol. 426, Issue 4-6
Nanopores and nucleic acids: prospects for ultrarapid sequencing
journal, April 2000
- Deamer, David W.; Akeson, Mark
- Trends in Biotechnology, Vol. 18, Issue 4, p. 147-151
Structure and Dynamics of Zeolites Investigated by Molecular Dynamics
journal, December 1997
- Demontis, Pierfranco; Suffritti, Giuseppe B.
- Chemical Reviews, Vol. 97, Issue 8
Rotational dynamics of simple asymmetric molecules
journal, February 2015
- Fragiadakis, D.; Roland, C. M.
- Physical Review E, Vol. 91, Issue 2
Hysteresis in the cyclic adsorption of acetone, ethanol and ethyl acetate on activated carbon
journal, January 2000
- Gales, L.; Mendes, A.; Costa, C.
- Carbon, Vol. 38, Issue 7
Molecular dynamics simulation study of meso-confined propane in TiO2
journal, September 2015
- Gautam, Siddharth; Cole, David
- Chemical Physics, Vol. 458
Diffusion of propylene adsorbed in Na-Y and Na-ZSM5 zeolites: Neutron scattering and FTIR studies
journal, November 2008
- Gautam, S.; Tripathi, A. K.; Kamble, V. S.
- Pramana, Vol. 71, Issue 5
Rotational dynamics of propylene in ZSM-5 zeolitic frameworks
journal, January 2011
- Gautam, Siddharth; Sharma, V. K.; Mitra, S.
- Chemical Physics Letters, Vol. 501, Issue 4-6
Structure and Dynamics of Confined C-O-H Fluids Relevant to the Subsurface: Application of Magnetic Resonance, Neutron Scattering, and Molecular Dynamics Simulations
journal, June 2017
- Gautam, Siddharth S.; Ok, Salim; Cole, David R.
- Frontiers in Earth Science, Vol. 5
Location dependent orientational structure and dynamics of ethane in ZSM5
journal, March 2016
- Gautam, Siddharth; Liu, Tingting; Patankar, Sumant
- Chemical Physics Letters, Vol. 648
Dynamics of n -Butane−Methane Mixtures in Silicalite, Using Quasielastic Neutron Scattering and Molecular Dynamics Simulations
journal, June 2000
- Gergidis, Leonidas N.; Theodorou, Doros N.; Jobic, Hervé
- The Journal of Physical Chemistry B, Vol. 104, Issue 23
Diffusion Anomaly as a Function of Molecular Length of Linear Molecules: Levitation Effect
journal, June 2003
- Ghorai, Pradip Kr.; Yashonath, Subramanian; Demontis, Pierfranco
- Journal of the American Chemical Society, Vol. 125, Issue 23
Effect of pressure on the phase behavior and structure of water confined between nanoscale hydrophobic and hydrophilic plates
journal, April 2006
- Giovambattista, Nicolas; Rossky, Peter J.; Debenedetti, Pablo G.
- Physical Review E, Vol. 73, Issue 4
Dynamic properties of propylene glycol confined in ZSM-5 zeolite matrix—A computer simulation study
journal, January 2012
- Górny, K.; Dendzik, Z.; Raczyński, P.
- Solid State Communications, Vol. 152, Issue 1
Non-Debye dipolar relaxation of ethylene glycol embedded in ZSM-5 zeolite host matrix — Computer simulation study
journal, March 2013
- Górny, K.; Dendzik, Z.; Pabiszczak, M.
- Journal of Non-Crystalline Solids, Vol. 364
Towards Enhanced Gas Sensor Performance with Fluoropolymer Membranes
journal, September 2016
- Graunke, Thorsten; Schmitt, Katrin; Raible, Stefan
- Sensors, Vol. 16, Issue 10
Effects of Confinement on Water Structure and Dynamics: A Molecular Simulation Study
journal, February 2007
- Hirunsit, P.; Balbuena, P. B.
- The Journal of Physical Chemistry C, Vol. 111, Issue 4
Transport properties of CO2-expanded acetonitrile from molecular dynamics simulations
journal, February 2007
- Houndonougbo, Yao; Laird, Brian B.; Kuczera, Krzysztof
- The Journal of Chemical Physics, Vol. 126, Issue 7
Molecular dynamics simulation of ethene diffusion in MFI and H[Al]ZSM-5
journal, November 1999
- Jianfen, F.; van de Graaf, B.; Xiao, H. M.
- Journal of Molecular Structure: THEOCHEM, Vol. 492, Issue 1-3
Mass transfer in mesoporous materials: the benefit of microscopic diffusion measurement
journal, January 2013
- Kärger, Jörg; Valiullin, Rustem
- Chemical Society Reviews, Vol. 42, Issue 9
Effects of confinement on the phase behavior of supercooled water
journal, March 1998
- Koga, Kenichiro; Zeng, X. C.; Tanaka, Hideki
- Chemical Physics Letters, Vol. 285, Issue 3-4
Structure of synthetic zeolite ZSM-5
journal, March 1978
- Kokotailo, G. T.; Lawton, S. L.; Olson, D. H.
- Nature, Vol. 272, Issue 5652
Does lattice vibration drive diffusion in zeolites?
journal, February 2001
- Kopelevich, Dmitry I.; Chang, Hsueh-Chia
- The Journal of Chemical Physics, Vol. 114, Issue 8
Thickness trends and sequence stratigraphy of the Middle Devonian Marcellus Formation, Appalachian Basin: Implications for Acadian foreland basin evolution
journal, January 2011
- Lash, Gary G.; Engelder, Terry
- AAPG Bulletin, Vol. 95, Issue 1
Hydrogen Storage in Single-Walled Carbon Nanotubes at Room Temperature
journal, November 1999
- Liu, C.
- Science, Vol. 286, Issue 5442
Transferable Potentials for Phase Equilibria. 1. United-Atom Description of n-Alkanes
March 1998
- Martin, Marcus G.; Siepmann, J. Ilja
Review: static and dynamic behavior of liquids inside carbon nanotubes
journal, April 2008
- Mattia, Davide; Gogotsi, Yury
- Microfluidics and Nanofluidics, Vol. 5, Issue 3
Effect of solute geometry and orientation on the rejection of uncharged compounds by nanofiltration
journal, June 2006
- Santos, José L. C.; de Beukelaar, Philip; Vankelecom, Ivo F. J.
- Separation and Purification Technology, Vol. 50, Issue 1
Shape Selectivity in Hydrocarbon Conversion
journal, February 2001
- Schenk, Merijn; Smit, Berend; Vlugt, Thijs J. H.
- Angewandte Chemie International Edition, Vol. 40, Issue 4
Dynamics of Adsorbed Hydrocarbon in Nanoporous Zeolite Framework
journal, June 2009
- Sharma, V. K.; Gautam, S.; Mitra, S.
- The Journal of Physical Chemistry B, Vol. 113, Issue 23
Dynamics of Propylene adsorbed in Na-Y and Na-ZSM5 Zeolites: A QENS and MD Simulation Study
journal, February 2010
- Sharma, V. K.; Gautam, S.; Mitra, S.
- Zeitschrift für Physikalische Chemie, Vol. 224, Issue 1-2
Towards a molecular understanding of shape selectivity
journal, February 2008
- Smit, Berend; Maesen, Theo L. M.
- Nature, Vol. 451, Issue 7179
Structure, Dynamics, and Phase Behavior of Water in TiO 2 Nanopores
journal, February 2013
- González Solveyra, Estefanía; de la Llave, Ezequiel; Molinero, Valeria
- The Journal of Physical Chemistry C, Vol. 117, Issue 7
Single Carbon Nanotube Membranes: A Well-Defined Model for Studying Mass Transport through Nanoporous Materials
journal, December 2000
- Sun, Li; Crooks, Richard M.
- Journal of the American Chemical Society, Vol. 122, Issue 49, p. 12340-12345
Why is the melting point of propane the lowest among n-alkanes?
journal, January 2000
- Thalladi, Venkat R.; Boese, Roland
- New Journal of Chemistry, Vol. 24, Issue 8
DL_POLY_3: new dimensions in molecular dynamics simulations via massive parallelism
journal, January 2006
- Todorov, Ilian T.; Smith, William; Trachenko, Kostya
- Journal of Materials Chemistry, Vol. 16, Issue 20, p. 1911-1918
Nanofiltration as a treatment method for the removal of pesticides from ground waters
journal, September 1998
- Van der Bruggen, B.; Schaep, J.; Maes, W.
- Desalination, Vol. 117, Issue 1-3
Influence of molecular size, polarity and charge on the retention of organic molecules by nanofiltration
journal, April 1999
- Van der Bruggen, B.; Schaep, J.; Wilms, D.
- Journal of Membrane Science, Vol. 156, Issue 1
On the location and disorder of the tetrapropylammonium (TPA) ion in zeolite ZSM-5 with improved framework accuracy
journal, April 1987
- van Koningsveld, H.; van Bekkum, H.; Jansen, J. C.
- Acta Crystallographica Section B Structural Science, Vol. 43, Issue 2
Molecular Structure of Water at Interfaces: Wetting at the Nanometer Scale
journal, April 2006
- Verdaguer, A.; Sacha, G. M.; Bluhm, H.
- Chemical Reviews, Vol. 106, Issue 4
The broadband rotational spectrum of fully deuterated acetaldehyde (CD3CDO) in a CW supersonic expansion
journal, December 2017
- Zaleski, Daniel P.; Duan, Chuanxi; Carvajal, Miguel
- Journal of Molecular Spectroscopy, Vol. 342
DNA-assisted dispersion and separation of carbon nanotubes
journal, April 2003
- Zheng, Ming; Jagota, Anand; Semke, Ellen D.
- Nature Materials, Vol. 2, Issue 5
Works referencing / citing this record:
Sorption, Structure and Dynamics of CO2 and Ethane in Silicalite at High Pressure: A Combined Monte Carlo and Molecular Dynamics Simulation Study
journal, December 2018
- Gautam, Siddharth; Liu, Tingting; Cole, David
- Molecules, Vol. 24, Issue 1
Figures / Tables found in this record:

 Search WorldCat to find libraries that may hold this journal
Search WorldCat to find libraries that may hold this journal