Molecular understanding of polyelectrolyte binders that actively regulate ion transport in sulfur cathodes
Abstract
Polymer binders in battery electrodes may be either active or passive. This distinction depends on whether the polymer influences charge or mass transport in the electrode. Although it is desirable to understand how to tailor the macromolecular design of a polymer to play a passive or active role, design rules are still lacking, as is a framework to assess the divergence in such behaviors. Here, we reveal the molecular-level underpinnings that distinguish an active polyelectrolyte binder designed for lithium–sulfur batteries from a passive alternative. The binder, a cationic polyelectrolyte, is shown to both facilitate lithium-ion transport through its reconfigurable network of mobile anions and restrict polysulfide diffusion from mesoporous carbon hosts by anion metathesis, which we show is selective for higher oligomers. Furthermore, these attributes allow cells to be operated for >100 cycles with excellent rate capability using cathodes with areal sulfur loadings up to 8.1mgcm–2.
- Authors:
-
- Lawrence Berkeley National Lab. (LBNL), Berkeley, CA (United States). Joint Center for Energy Storage Research
- Lawrence Berkeley National Lab. (LBNL), Berkeley, CA (United States). Molecular Foundry
- Argonne National Lab. (ANL), Argonne, IL (United States). Joint Center for Energy Storage Research
- Massachusetts Inst. of Technology (MIT), Cambridge, MA (United States). Dept. of Materials Science and Engineering
- Univ. of California, Berkeley, CA (United States). Dept. of Chemistry
- Lawrence Berkeley National Lab. (LBNL), Berkeley, CA (United States). Joint Center for Energy Storage Research, Molecular Foundry
- Publication Date:
- Research Org.:
- Lawrence Berkeley National Laboratory (LBNL), Berkeley, CA (United States); Argonne National Laboratory (ANL), Argonne, IL (United States)
- Sponsoring Org.:
- SC-22.2 USDOE Office of Science (SC), Basic Energy Sciences (BES) (SC-22). Materials Sciences & Engineering Division; USDOE Office of Science (SC), Office of Basic Energy Sciences (BES) (SC-22). Joint Center for Energy Storage Research (JCESR); USDOE Office of Science (SC), National Energy Research Scientific Computing Center (NERSC); USDOE Office of Science (SC), Basic Energy Sciences (BES). Scientific User Facilities Division
- OSTI Identifier:
- 1417620
- Alternate Identifier(s):
- OSTI ID: 1489228
- Grant/Contract Number:
- AC02-05CH11231; AC02-06CH11357
- Resource Type:
- Accepted Manuscript
- Journal Name:
- Nature Communications
- Additional Journal Information:
- Journal Volume: 8; Journal Issue: 1; Journal ID: ISSN 2041-1723
- Publisher:
- Nature Publishing Group
- Country of Publication:
- United States
- Language:
- English
- Subject:
- 36 MATERIALS SCIENCE; 25 ENERGY STORAGE; batteries; polymer characterization
Citation Formats
Li, Longjun, Pascal, Tod A., Connell, Justin G., Fan, Frank Y., Meckler, Stephen M., Ma, Lin, Chiang, Yet-Ming, Prendergast, David, and Helms, Brett A. Molecular understanding of polyelectrolyte binders that actively regulate ion transport in sulfur cathodes. United States: N. p., 2017.
Web. doi:10.1038/s41467-017-02410-6.
Li, Longjun, Pascal, Tod A., Connell, Justin G., Fan, Frank Y., Meckler, Stephen M., Ma, Lin, Chiang, Yet-Ming, Prendergast, David, & Helms, Brett A. Molecular understanding of polyelectrolyte binders that actively regulate ion transport in sulfur cathodes. United States. https://doi.org/10.1038/s41467-017-02410-6
Li, Longjun, Pascal, Tod A., Connell, Justin G., Fan, Frank Y., Meckler, Stephen M., Ma, Lin, Chiang, Yet-Ming, Prendergast, David, and Helms, Brett A. Fri .
"Molecular understanding of polyelectrolyte binders that actively regulate ion transport in sulfur cathodes". United States. https://doi.org/10.1038/s41467-017-02410-6. https://www.osti.gov/servlets/purl/1417620.
@article{osti_1417620,
title = {Molecular understanding of polyelectrolyte binders that actively regulate ion transport in sulfur cathodes},
author = {Li, Longjun and Pascal, Tod A. and Connell, Justin G. and Fan, Frank Y. and Meckler, Stephen M. and Ma, Lin and Chiang, Yet-Ming and Prendergast, David and Helms, Brett A.},
abstractNote = {Polymer binders in battery electrodes may be either active or passive. This distinction depends on whether the polymer influences charge or mass transport in the electrode. Although it is desirable to understand how to tailor the macromolecular design of a polymer to play a passive or active role, design rules are still lacking, as is a framework to assess the divergence in such behaviors. Here, we reveal the molecular-level underpinnings that distinguish an active polyelectrolyte binder designed for lithium–sulfur batteries from a passive alternative. The binder, a cationic polyelectrolyte, is shown to both facilitate lithium-ion transport through its reconfigurable network of mobile anions and restrict polysulfide diffusion from mesoporous carbon hosts by anion metathesis, which we show is selective for higher oligomers. Furthermore, these attributes allow cells to be operated for >100 cycles with excellent rate capability using cathodes with areal sulfur loadings up to 8.1mgcm–2.},
doi = {10.1038/s41467-017-02410-6},
journal = {Nature Communications},
number = 1,
volume = 8,
place = {United States},
year = {Fri Dec 22 00:00:00 EST 2017},
month = {Fri Dec 22 00:00:00 EST 2017}
}
Web of Science
Figures / Tables:
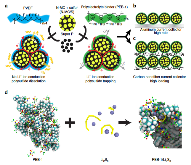 Fig. 1: Illustration of the fabrication of sulfur electrodes with PVDF or PEB-1 binder. a The cathode is comprised of sulfur-active materials loaded into N-doped mesoporous carbon (N-MC) hosts, ‘Super P’ as the conductive additive, and a polymer binder (PEB-1 or PVDF). b A conventional sulfur cathode cast onto anmore »
Fig. 1: Illustration of the fabrication of sulfur electrodes with PVDF or PEB-1 binder. a The cathode is comprised of sulfur-active materials loaded into N-doped mesoporous carbon (N-MC) hosts, ‘Super P’ as the conductive additive, and a polymer binder (PEB-1 or PVDF). b A conventional sulfur cathode cast onto anmore »
Works referenced in this record:
Investigation of surface effects through the application of the functional binders in lithium sulfur batteries
journal, September 2015
- Ai, Guo; Dai, Yiling; Ye, Yifan
- Nano Energy, Vol. 16
An Elastic, Conductive, Electroactive Nanocomposite Binder for Flexible Sulfur Cathodes in Lithium-Sulfur Batteries
journal, September 2016
- Milroy, Craig; Manthiram, Arumugam
- Advanced Materials, Vol. 28, Issue 44
A high performance lithium-ion sulfur battery based on a Li 2 S cathode using a dual-phase electrolyte
journal, January 2015
- Wang, Lina; Wang, Yonggang; Xia, Yongyao
- Energy & Environmental Science, Vol. 8, Issue 5
Insights into Li-S Battery Cathode Capacity Fading Mechanisms: Irreversible Oxidation of Active Mass during Cycling
journal, January 2012
- Diao, Yan; Xie, Kai; Xiong, Shizhao
- Journal of The Electrochemical Society, Vol. 159, Issue 11
Why PEO as a binder or polymer coating increases capacity in the Li–S system
journal, January 2013
- Lacey, Matthew J.; Jeschull, Fabian; Edström, Kristina
- Chemical Communications, Vol. 49, Issue 76
Fingerprinting Lithium-Sulfur Battery Reaction Products by X-ray Absorption Spectroscopy
journal, January 2014
- Wujcik, Kevin H.; Velasco-Velez, Juan; Wu, Cheng Hao
- Journal of The Electrochemical Society, Vol. 161, Issue 6
Pyrrolidinium-based polymeric ionic liquids as mechanically and electrochemically stable polymer electrolytes
journal, March 2009
- Pont, Anne-Laure; Marcilla, Rebeca; De Meatza, Iratxe
- Journal of Power Sources, Vol. 188, Issue 2
Structural Factors of Sulfur Cathodes with Poly(ethylene oxide) Binder for Performance of Rechargeable Lithium Sulfur Batteries
journal, January 2002
- Cheon, Sang-Eun; Cho, Ji-Hoon; Ko, Ki-Seok
- Journal of The Electrochemical Society, Vol. 149, Issue 11, p. A1437-A1441
Enhanced Cyclability for Sulfur Cathode Achieved by a Water-Soluble Binder
journal, July 2011
- He, Min; Yuan, Li-Xia; Zhang, Wu-Xing
- The Journal of Physical Chemistry C, Vol. 115, Issue 31
Layer-by-Layer Assembled C/S Cathode with Trace Binder for Li–S Battery Application
journal, November 2015
- Wang, Qian; Yan, Na; Wang, Meiri
- ACS Applied Materials & Interfaces, Vol. 7, Issue 45
Elemental-Sulfur-Mediated Facile Synthesis of a Covalent Triazine Framework for High-Performance Lithium-Sulfur Batteries
journal, January 2016
- Talapaneni, Siddulu Naidu; Hwang, Tae Hoon; Je, Sang Hyun
- Angewandte Chemie, Vol. 128, Issue 9
Elemental Sulfur and Molybdenum Disulfide Composites for Li–S Batteries with Long Cycle Life and High-Rate Capability
journal, May 2016
- Dirlam, Philip T.; Park, Jungjin; Simmonds, Adam G.
- ACS Applied Materials & Interfaces, Vol. 8, Issue 21
Ionic liquid-enhanced solid state electrolyte interface (SEI) for lithium–sulfur batteries
journal, January 2013
- Zheng, Jianming; Gu, Meng; Chen, Honghao
- Journal of Materials Chemistry A, Vol. 1, Issue 29
Phosphorous Pentasulfide as a Novel Additive for High-Performance Lithium-Sulfur Batteries
journal, June 2012
- Lin, Zhan; Liu, Zengcai; Fu, Wujun
- Advanced Functional Materials, Vol. 23, Issue 8
Strong Sulfur Binding with Conducting Magnéli-Phase Ti n O 2 n –1 Nanomaterials for Improving Lithium–Sulfur Batteries
journal, August 2014
- Tao, Xinyong; Wang, Jianguo; Ying, Zhuogao
- Nano Letters, Vol. 14, Issue 9
Li-S Battery Analyzed by UV/Vis in Operando Mode
journal, June 2013
- Patel, Manu U. M.; Demir-Cakan, Rezan; Morcrette, Mathieu
- ChemSusChem, Vol. 6, Issue 7
Unique behaviour of nonsolvents for polysulphides in lithium–sulphur batteries
journal, January 2014
- Cuisinier, M.; Cabelguen, P. -E.; Adams, B. D.
- Energy Environ. Sci., Vol. 7, Issue 8
Electrochemical performance of lithium/sulfur cells with three different polymer electrolytes
journal, August 2000
- Marmorstein, D.; Yu, T. H.; Striebel, K. A.
- Journal of Power Sources, Vol. 89, Issue 2
Moving to a Solid-State Configuration: A Valid Approach to Making Lithium-Sulfur Batteries Viable for Practical Applications
journal, September 2010
- Hassoun, Jusef; Scrosati, Bruno
- Advanced Materials, Vol. 22, Issue 45, p. 5198-5201
Lithium Iodide as a Promising Electrolyte Additive for Lithium-Sulfur Batteries: Mechanisms of Performance Enhancement
journal, November 2014
- Wu, Feixiang; Lee, Jung Tae; Nitta, Naoki
- Advanced Materials, Vol. 27, Issue 1
Sulfur Speciation in Li–S Batteries Determined by Operando X-ray Absorption Spectroscopy
journal, September 2013
- Cuisinier, Marine; Cabelguen, Pierre-Etienne; Evers, Scott
- The Journal of Physical Chemistry Letters, Vol. 4, Issue 19
Rechargeable Lithium–Sulfur Batteries
journal, July 2014
- Manthiram, Arumugam; Fu, Yongzhu; Chung, Sheng-Heng
- Chemical Reviews, Vol. 114, Issue 23
Nanomaterials: Science and applications in the lithium–sulfur battery
journal, June 2015
- Ma, Lin; Hendrickson, Kenville E.; Wei, Shuya
- Nano Today, Vol. 10, Issue 3
Application of gelatin as a binder for the sulfur cathode in lithium–sulfur batteries
journal, October 2008
- Sun, Jing; Huang, Yaqin; Wang, Weikun
- Electrochimica Acta, Vol. 53, Issue 24, p. 7084-7088
Binder Based on Polyelectrolyte for High Capacity Density Lithium/Sulfur Battery
journal, January 2012
- Zhang, Sheng S.
- Journal of The Electrochemical Society, Vol. 159, Issue 8
A new class of Solvent-in-Salt electrolyte for high-energy rechargeable metallic lithium batteries
journal, February 2013
- Suo, Liumin; Hu, Yong-Sheng; Li, Hong
- Nature Communications, Vol. 4, Issue 1
Hierarchical pore-in-pore and wire-in-wire catalysts for rechargeable Zn– and Li–air batteries with ultra-long cycle life and high cell efficiency
journal, January 2015
- Li, Longjun; Liu, Chao; He, Guang
- Energy & Environmental Science, Vol. 8, Issue 11
A multi functional binder with lithium ion conductive polymer and polysulfide absorbents to improve cycleability of lithium–sulfur batteries
journal, October 2015
- Li, Gaoran; Cai, Wenlong; Liu, Binhong
- Journal of Power Sources, Vol. 294
Attainable Gravimetric and Volumetric Energy Density of Li–S and Li Ion Battery Cells with Solid Separator-Protected Li Metal Anodes
journal, October 2015
- McCloskey, Bryan D.
- The Journal of Physical Chemistry Letters, Vol. 6, Issue 22
X-ray Absorption Spectra of Dissolved Polysulfides in Lithium–Sulfur Batteries from First-Principles
journal, April 2014
- Pascal, Tod A.; Wujcik, Kevin H.; Velasco-Velez, Juan
- The Journal of Physical Chemistry Letters, Vol. 5, Issue 9
Ionic-Liquid-Based Polymer Electrolytes for Battery Applications
journal, November 2015
- Osada, Irene; de Vries, Henrik; Scrosati, Bruno
- Angewandte Chemie International Edition, Vol. 55, Issue 2
Effect of Sulfur Impurities on Li∕TiS[sub 2] Cells
journal, January 1981
- Rao, B. M. L.
- Journal of The Electrochemical Society, Vol. 128, Issue 5
Enhanced Electrochemical Kinetics on Conductive Polar Mediators for Lithium-Sulfur Batteries
journal, October 2016
- Peng, Hong-Jie; Zhang, Ge; Chen, Xiang
- Angewandte Chemie International Edition, Vol. 55, Issue 42
Determination of Ion Cluster Sizes and Cluster-to-Cluster Distances in Ionomers by Four-Pulse Double Electron Electron Resonance Spectroscopy
journal, October 2000
- Pannier, M.; Schädler, V.; Schöps, M.
- Macromolecules, Vol. 33, Issue 21
Synthesis of three-dimensionally interconnected sulfur-rich polymers for cathode materials of high-rate lithium–sulfur batteries
journal, June 2015
- Kim, Hoon; Lee, Joungphil; Ahn, Hyungmin
- Nature Communications, Vol. 6, Issue 1
Effect of Sulfur Impurities on Li∕TiS[sub 2] Cells
journal, January 1981
- Rao, B. M. L.
- Journal of The Electrochemical Society, Vol. 128, Issue 5
A Graphene-Pure-Sulfur Sandwich Structure for Ultrafast, Long-Life Lithium-Sulfur Batteries
journal, November 2013
- Zhou, Guangmin; Pei, Songfeng; Li, Lu
- Advanced Materials, Vol. 26, Issue 4
Mechanism and Kinetics of Li 2 S Precipitation in Lithium-Sulfur Batteries
journal, August 2015
- Fan, Frank Y.; Carter, W. Craig; Chiang, Yet-Ming
- Advanced Materials, Vol. 27, Issue 35
A Family of Highly Ordered Mesoporous Polymer Resin and Carbon Structures from Organic−Organic Self-Assembly
journal, September 2006
- Meng, Yan; Gu, Dong; Zhang, Fuqiang
- Chemistry of Materials, Vol. 18, Issue 18
A highly ordered nanostructured carbon–sulphur cathode for lithium–sulphur batteries
journal, May 2009
- Ji, Xiulei; Lee, Kyu Tae; Nazar, Linda F.
- Nature Materials, Vol. 8, Issue 6, p. 500-506
Elemental-Sulfur-Mediated Facile Synthesis of a Covalent Triazine Framework for High-Performance Lithium-Sulfur Batteries
journal, January 2016
- Talapaneni, Siddulu Naidu; Hwang, Tae Hoon; Je, Sang Hyun
- Angewandte Chemie International Edition, Vol. 55, Issue 9
The synergetic effect of lithium polysulfide and lithium nitrate to prevent lithium dendrite growth
journal, June 2015
- Li, Weiyang; Yao, Hongbin; Yan, Kai
- Nature Communications, Vol. 6, Issue 1
Three-Dimensional Growth of Li 2 S in Lithium–Sulfur Batteries Promoted by a Redox Mediator
journal, December 2015
- Gerber, Laura C. H.; Frischmann, Peter D.; Fan, Frank Y.
- Nano Letters, Vol. 16, Issue 1
Nitrogen-Doped Mesoporous Carbon Promoted Chemical Adsorption of Sulfur and Fabrication of High-Areal-Capacity Sulfur Cathode with Exceptional Cycling Stability for Lithium-Sulfur Batteries
journal, October 2013
- Song, Jiangxuan; Xu, Terrence; Gordin, Mikhail L.
- Advanced Functional Materials, Vol. 24, Issue 9
Rechargeable Lithium–Sulfur Batteries
journal, July 2014
- Manthiram, Arumugam; Fu, Yongzhu; Chung, Sheng-Heng
- Chemical Reviews, Vol. 114, Issue 23
Sparingly Solvating Electrolytes for High Energy Density Lithium–Sulfur Batteries
journal, August 2016
- Cheng, Lei; Curtiss, Larry A.; Zavadil, Kevin R.
- ACS Energy Letters, Vol. 1, Issue 3
Recent Advances in Electrolytes for Lithium-Sulfur Batteries
journal, April 2015
- Zhang, Shiguo; Ueno, Kazuhide; Dokko, Kaoru
- Advanced Energy Materials, Vol. 5, Issue 16
Sulfur Speciation in Li–S Batteries Determined by Operando X-ray Absorption Spectroscopy
journal, September 2013
- Cuisinier, Marine; Cabelguen, Pierre-Etienne; Evers, Scott
- The Journal of Physical Chemistry Letters, Vol. 4, Issue 19
Thermodynamic origins of the solvent-dependent stability of lithium polysulfides from first principles
journal, January 2017
- Pascal, Tod A.; Wujcik, Kevin H.; Wang, Dunyang Rita
- Physical Chemistry Chemical Physics, Vol. 19, Issue 2
Polysulfide-Blocking Microporous Polymer Membrane Tailored for Hybrid Li-Sulfur Flow Batteries
journal, August 2015
- Li, Changyi; Ward, Ashleigh L.; Doris, Sean E.
- Nano Letters, Vol. 15, Issue 9
A Graphene-Pure-Sulfur Sandwich Structure for Ultrafast, Long-Life Lithium-Sulfur Batteries
journal, November 2013
- Zhou, Guangmin; Pei, Songfeng; Li, Lu
- Advanced Materials, Vol. 26, Issue 4
Metal–organic framework-based separator for lithium–sulfur batteries
journal, June 2016
- Bai, Songyan; Liu, Xizheng; Zhu, Kai
- Nature Energy, Vol. 1, Issue 7
Highly Reversible Lithium/Dissolved Polysulfide Batteries with Carbon Nanotube Electrodes
journal, May 2013
- Fu, Yongzhu; Su, Yu-Sheng; Manthiram, Arumugam
- Angewandte Chemie, Vol. 125, Issue 27, p. 7068-7073
Physical Structure of Ionomers
journal, April 1968
- Longworth, R.; Vaughan, Daniel J.
- Nature, Vol. 218, Issue 5136
Observation of cluster formation in an ionomer
journal, December 1987
- Galambos, A. F.; Stockton, W. B.; Koberstein, J. T.
- Macromolecules, Vol. 20, Issue 12
Enhanced Electrochemical Kinetics on Conductive Polar Mediators for Lithium-Sulfur Batteries
journal, October 2016
- Peng, Hong-Jie; Zhang, Ge; Chen, Xiang
- Angewandte Chemie, Vol. 128, Issue 42
Application of gelatin as a binder for the sulfur cathode in lithium–sulfur batteries
journal, October 2008
- Sun, Jing; Huang, Yaqin; Wang, Weikun
- Electrochimica Acta, Vol. 53, Issue 24, p. 7084-7088
Direct observation of lithium polysulfides in lithium–sulfur batteries using operando X-ray diffraction
journal, May 2017
- Conder, Joanna; Bouchet, Renaud; Trabesinger, Sigita
- Nature Energy, Vol. 2, Issue 6
Energy Storage Materials Synthesized from Ionic Liquids
journal, October 2014
- Gebresilassie Eshetu, Gebrekidan; Armand, Michel; Scrosati, Bruno
- Angewandte Chemie International Edition, Vol. 53, Issue 49
Ionic liquid-enhanced solid state electrolyte interface (SEI) for lithium–sulfur batteries
journal, January 2013
- Zheng, Jianming; Gu, Meng; Chen, Honghao
- Journal of Materials Chemistry A, Vol. 1, Issue 29
Carbonyl- β -Cyclodextrin as a Novel Binder for Sulfur Composite Cathodes in Rechargeable Lithium Batteries
journal, October 2012
- Wang, Jiulin; Yao, Zhendong; Monroe, Charles W.
- Advanced Functional Materials, Vol. 23, Issue 9
Achieving high capacity retention in lithium-sulfur batteries with an aqueous binder
journal, November 2016
- Lu, Yan-Qiu; Li, Jun-Tao; Peng, Xin-Xing
- Electrochemistry Communications, Vol. 72
Simultaneous Conduction of Electronic Charge and Lithium Ions in Block Copolymers
journal, January 2012
- Patel, Shrayesh N.; Javier, Anna E.; Stone, Greg M.
- ACS Nano, Vol. 6, Issue 2
On the dispersion of lithium-sulfur battery cathode materials effected by electrostatic and stereo-chemical factors of binders
journal, August 2016
- Hong, Xiaoheng; Jin, Jun; Wen, Zhaoyin
- Journal of Power Sources, Vol. 324
Moving to a Solid-State Configuration: A Valid Approach to Making Lithium-Sulfur Batteries Viable for Practical Applications
journal, September 2010
- Hassoun, Jusef; Scrosati, Bruno
- Advanced Materials, Vol. 22, Issue 45, p. 5198-5201
Stable lithium electrodeposition in liquid and nanoporous solid electrolytes
journal, August 2014
- Lu, Yingying; Tu, Zhengyuan; Archer, Lynden A.
- Nature Materials, Vol. 13, Issue 10
Critical Link between Materials Chemistry and Cell-Level Design for High Energy Density and Low Cost Lithium-Sulfur Transportation Battery
journal, January 2015
- Eroglu, Damla; Zavadil, Kevin R.; Gallagher, Kevin G.
- Journal of The Electrochemical Society, Vol. 162, Issue 6
The use of elemental sulfur as an alternative feedstock for polymeric materials
journal, April 2013
- Chung, Woo Jin; Griebel, Jared J.; Kim, Eui Tae
- Nature Chemistry, Vol. 5, Issue 6
Insights into Li-S Battery Cathode Capacity Fading Mechanisms: Irreversible Oxidation of Active Mass during Cycling
journal, January 2012
- Diao, Yan; Xie, Kai; Xiong, Shizhao
- Journal of The Electrochemical Society, Vol. 159, Issue 11
Structural Factors of Sulfur Cathodes with Poly(ethylene oxide) Binder for Performance of Rechargeable Lithium Sulfur Batteries
journal, January 2002
- Cheon, Sang-Eun; Cho, Ji-Hoon; Ko, Ki-Seok
- Journal of The Electrochemical Society, Vol. 149, Issue 11, p. A1437-A1441
QTPIE: Charge transfer with polarization current equalization. A fluctuating charge model with correct asymptotics
journal, April 2007
- Chen, Jiahao; Martínez, Todd J.
- Chemical Physics Letters, Vol. 438, Issue 4-6
Why PEO as a binder or polymer coating increases capacity in the Li–S system
journal, January 2013
- Lacey, Matthew J.; Jeschull, Fabian; Edström, Kristina
- Chemical Communications, Vol. 49, Issue 76
Strong Sulfur Binding with Conducting Magnéli-Phase Ti n O 2 n –1 Nanomaterials for Improving Lithium–Sulfur Batteries
journal, August 2014
- Tao, Xinyong; Wang, Jianguo; Ying, Zhuogao
- Nano Letters, Vol. 14, Issue 9
Attainable Gravimetric and Volumetric Energy Density of Li–S and Li Ion Battery Cells with Solid Separator-Protected Li Metal Anodes
journal, October 2015
- McCloskey, Bryan D.
- The Journal of Physical Chemistry Letters, Vol. 6, Issue 22
Fingerprinting Lithium-Sulfur Battery Reaction Products by X-ray Absorption Spectroscopy
journal, January 2014
- Wujcik, Kevin H.; Velasco-Velez, Juan; Wu, Cheng Hao
- Journal of The Electrochemical Society, Vol. 161, Issue 6
Investigation of surface effects through the application of the functional binders in lithium sulfur batteries
journal, September 2015
- Ai, Guo; Dai, Yiling; Ye, Yifan
- Nano Energy, Vol. 16
Charge equilibration for molecular dynamics simulations
journal, April 1991
- Rappe, Anthony K.; Goddard, William A.
- The Journal of Physical Chemistry, Vol. 95, Issue 8
Highly reversible Li/dissolved polysulfide batteries with binder-free carbon nanofiber electrodes
journal, January 2013
- Zu, Chenxi; Fu, Yongzhu; Manthiram, Arumugam
- Journal of Materials Chemistry A, Vol. 1, Issue 35
Introducing ion-transport-regulating nanochannels to lithium-sulfur batteries
journal, March 2017
- Pan, Yuede; Zhou, Yahong; Zhao, Qing
- Nano Energy, Vol. 33
Stable Lithium Electrodeposition in Liquid and Nanoporous Solid Electrolytes
text, January 2014
- Lu, Yingying; Tu, Zhengyuan; Archer, Lynden
- arXiv
Elemental Sulfur and Molybdenum Disulfide Composites for Li–S Batteries with Long Cycle Life and High-Rate Capability
journal, May 2016
- Dirlam, Philip T.; Park, Jungjin; Simmonds, Adam G.
- ACS Applied Materials & Interfaces, Vol. 8, Issue 21
Layer-by-Layer Assembled C/S Cathode with Trace Binder for Li–S Battery Application
journal, November 2015
- Wang, Qian; Yan, Na; Wang, Meiri
- ACS Applied Materials & Interfaces, Vol. 7, Issue 45
Nanomaterials: Science and applications in the lithium–sulfur battery
journal, June 2015
- Ma, Lin; Hendrickson, Kenville E.; Wei, Shuya
- Nano Today, Vol. 10, Issue 3
Pyrrolidinium-based polymeric ionic liquids as mechanically and electrochemically stable polymer electrolytes
journal, March 2009
- Pont, Anne-Laure; Marcilla, Rebeca; De Meatza, Iratxe
- Journal of Power Sources, Vol. 188, Issue 2
Structural and chemical synergistic encapsulation of polysulfides enables ultralong-life lithium–sulfur batteries
journal, January 2016
- Wang, Xiaolei; Li, Ge; Li, Jingde
- Energy & Environmental Science, Vol. 9, Issue 8
Metadynamics: a method to simulate rare events and reconstruct the free energy in biophysics, chemistry and material science
journal, November 2008
- Laio, Alessandro; Gervasio, Francesco L.
- Reports on Progress in Physics, Vol. 71, Issue 12
Highly Reversible Lithium/Dissolved Polysulfide Batteries with Carbon Nanotube Electrodes
journal, May 2013
- Fu, Yongzhu; Su, Yu-Sheng; Manthiram, Arumugam
- Angewandte Chemie, Vol. 125, Issue 27, p. 7068-7073
Rational Sulfur Cathode Design for Lithium–Sulfur Batteries: Sulfur-Embedded Benzoxazine Polymers
journal, August 2016
- Je, Sang Hyun; Hwang, Tae Hoon; Talapaneni, Siddulu Naidu
- ACS Energy Letters, Vol. 1, Issue 3
Enhanced Li–S Batteries Using Amine-Functionalized Carbon Nanotubes in the Cathode
journal, December 2015
- Ma, Lin; Zhuang, Houlong L.; Wei, Shuya
- ACS Nano, Vol. 10, Issue 1
A New Type of Multifunctional Polar Binder: Toward Practical Application of High Energy Lithium Sulfur Batteries
journal, February 2017
- Chen, Wei; Qian, Tao; Xiong, Jie
- Advanced Materials, Vol. 29, Issue 12
Advances in lithium–sulfur batteries based on multifunctional cathodes and electrolytes
journal, September 2016
- Pang, Quan; Liang, Xiao; Kwok, Chun Yuen
- Nature Energy, Vol. 1, Issue 9
Polysulfide-Blocking Microporous Polymer Membrane Tailored for Hybrid Li-Sulfur Flow Batteries
journal, August 2015
- Li, Changyi; Ward, Ashleigh L.; Doris, Sean E.
- Nano Letters, Vol. 15, Issue 9
Interpretation of small-angle x-ray and neutron scattering data for perfluorosulfonated ionomer membranes
journal, August 1986
- Kumar, Satish; Pineri, Michel
- Journal of Polymer Science Part B: Polymer Physics, Vol. 24, Issue 8
Li-S Battery Analyzed by UV/Vis in Operando Mode
journal, June 2013
- Patel, Manu U. M.; Demir-Cakan, Rezan; Morcrette, Mathieu
- ChemSusChem, Vol. 6, Issue 7
Porosity Blocking in Highly Porous Carbon Black by PVdF Binder and Its Implications for the Li–S System
journal, October 2014
- Lacey, Matthew J.; Jeschull, Fabian; Edström, Kristina
- The Journal of Physical Chemistry C, Vol. 118, Issue 45
Poly(ionic liquid)s: An update
journal, July 2013
- Yuan, Jiayin; Mecerreyes, David; Antonietti, Markus
- Progress in Polymer Science, Vol. 38, Issue 7
Enhanced Electrochemical Kinetics on Conductive Polar Mediators for Lithium-Sulfur Batteries
journal, October 2016
- Peng, Hong-Jie; Zhang, Ge; Chen, Xiang
- Angewandte Chemie, Vol. 128, Issue 42
Long-Life and High-Areal-Capacity Li–S Batteries Enabled by a Light-Weight Polar Host with Intrinsic Polysulfide Adsorption
journal, March 2016
- Pang, Quan; Nazar, Linda F.
- ACS Nano, Vol. 10, Issue 4
Phosphorous Pentasulfide as a Novel Additive for High-Performance Lithium-Sulfur Batteries
journal, June 2012
- Lin, Zhan; Liu, Zengcai; Fu, Wujun
- Advanced Functional Materials, Vol. 23, Issue 8
X-ray spectroscopy as a probe for lithium polysulfide radicals
journal, January 2015
- Pascal, Tod A.; Pemmaraju, C. D.; Prendergast, David
- Physical Chemistry Chemical Physics, Vol. 17, Issue 12
Highly Reversible Lithium/Dissolved Polysulfide Batteries with Carbon Nanotube Electrodes
journal, May 2013
- Fu, Yongzhu; Su, Yu-Sheng; Manthiram, Arumugam
- Angewandte Chemie International Edition, Vol. 52, Issue 27
On the Surface Chemical Aspects of Very High Energy Density, Rechargeable Li–Sulfur Batteries
journal, January 2009
- Aurbach, Doron; Pollak, Elad; Elazari, Ran
- Journal of The Electrochemical Society, Vol. 156, Issue 8, p. A694-A702
Determination of Ion Cluster Sizes and Cluster-to-Cluster Distances in Ionomers by Four-Pulse Double Electron Electron Resonance Spectroscopy
journal, October 2000
- Pannier, M.; Schädler, V.; Schöps, M.
- Macromolecules, Vol. 33, Issue 21
Critical Link between Materials Chemistry and Cell-Level Design for High Energy Density and Low Cost Lithium-Sulfur Transportation Battery
journal, January 2015
- Eroglu, Damla; Zavadil, Kevin R.; Gallagher, Kevin G.
- Journal of The Electrochemical Society, Vol. 162, Issue 6
Mechanism and Kinetics of Li 2 S Precipitation in Lithium-Sulfur Batteries
journal, August 2015
- Fan, Frank Y.; Carter, W. Craig; Chiang, Yet-Ming
- Advanced Materials, Vol. 27, Issue 35
A Lightweight TiO 2 /Graphene Interlayer, Applied as a Highly Effective Polysulfide Absorbent for Fast, Long-Life Lithium-Sulfur Batteries
journal, March 2015
- Xiao, Zhubing; Yang, Zhi; Wang, Lu
- Advanced Materials, Vol. 27, Issue 18
Simultaneous Conduction of Electronic Charge and Lithium Ions in Block Copolymers
journal, January 2012
- Patel, Shrayesh N.; Javier, Anna E.; Stone, Greg M.
- ACS Nano, Vol. 6, Issue 2
Low-Cost Higher Loading of a Sulfur Cathode
journal, December 2015
- Zhou, Weidong; Guo, Bingkun; Gao, Hongcai
- Advanced Energy Materials, Vol. 6, Issue 5
Surface-enhanced redox chemistry of polysulphides on a metallic and polar host for lithium-sulphur batteries
journal, August 2014
- Pang, Quan; Kundu, Dipan; Cuisinier, Marine
- Nature Communications, Vol. 5, Issue 1
Stable cycling of lithium sulfide cathodes through strong affinity with a bifunctional binder
journal, January 2013
- Seh, Zhi Wei; Zhang, Qianfan; Li, Weiyang
- Chemical Science, Vol. 4, Issue 9
On the dispersion of lithium-sulfur battery cathode materials effected by electrostatic and stereo-chemical factors of binders
journal, August 2016
- Hong, Xiaoheng; Jin, Jun; Wen, Zhaoyin
- Journal of Power Sources, Vol. 324
Rational Sulfur Cathode Design for Lithium–Sulfur Batteries: Sulfur-Embedded Benzoxazine Polymers
journal, August 2016
- Je, Sang Hyun; Hwang, Tae Hoon; Talapaneni, Siddulu Naidu
- ACS Energy Letters, Vol. 1, Issue 3
The use of elemental sulfur as an alternative feedstock for polymeric materials
journal, April 2013
- Chung, Woo Jin; Griebel, Jared J.; Kim, Eui Tae
- Nature Chemistry, Vol. 5, Issue 6
Ionic-Liquid-Based Polymer Electrolytes for Battery Applications
journal, November 2015
- Osada, Irene; de Vries, Henrik; Scrosati, Bruno
- Angewandte Chemie International Edition, Vol. 55, Issue 2
Observation of cluster formation in an ionomer
journal, December 1987
- Galambos, A. F.; Stockton, W. B.; Koberstein, J. T.
- Macromolecules, Vol. 20, Issue 12
Achieving high capacity retention in lithium-sulfur batteries with an aqueous binder
journal, November 2016
- Lu, Yan-Qiu; Li, Jun-Tao; Peng, Xin-Xing
- Electrochemistry Communications, Vol. 72
Porosity Blocking in Highly Porous Carbon Black by PVdF Binder and Its Implications for the Li–S System
journal, October 2014
- Lacey, Matthew J.; Jeschull, Fabian; Edström, Kristina
- The Journal of Physical Chemistry C, Vol. 118, Issue 45
Carbonyl- β -Cyclodextrin as a Novel Binder for Sulfur Composite Cathodes in Rechargeable Lithium Batteries
journal, October 2012
- Wang, Jiulin; Yao, Zhendong; Monroe, Charles W.
- Advanced Functional Materials, Vol. 23, Issue 9
X-ray Absorption Spectra of Dissolved Polysulfides in Lithium–Sulfur Batteries from First-Principles
journal, April 2014
- Pascal, Tod A.; Wujcik, Kevin H.; Velasco-Velez, Juan
- The Journal of Physical Chemistry Letters, Vol. 5, Issue 9
Recent Advances in Electrolytes for Lithium-Sulfur Batteries
journal, April 2015
- Zhang, Shiguo; Ueno, Kazuhide; Dokko, Kaoru
- Advanced Energy Materials, Vol. 5, Issue 16
A multi functional binder with lithium ion conductive polymer and polysulfide absorbents to improve cycleability of lithium–sulfur batteries
journal, October 2015
- Li, Gaoran; Cai, Wenlong; Liu, Binhong
- Journal of Power Sources, Vol. 294
Metadynamics: a method to simulate rare events and reconstruct the free energy in biophysics, chemistry and material science
journal, November 2008
- Laio, Alessandro; Gervasio, Francesco L.
- Reports on Progress in Physics, Vol. 71, Issue 12
Nitrogen-Doped Mesoporous Carbon Promoted Chemical Adsorption of Sulfur and Fabrication of High-Areal-Capacity Sulfur Cathode with Exceptional Cycling Stability for Lithium-Sulfur Batteries
journal, October 2013
- Song, Jiangxuan; Xu, Terrence; Gordin, Mikhail L.
- Advanced Functional Materials, Vol. 24, Issue 9
Stable Lithium Electrodeposition in Liquid and Nanoporous Solid Electrolytes
text, January 2014
- Lu, Yingying; Tu, Zhengyuan; Archer, Lynden
- arXiv
Beamline 10.3.2 at ALS: a hard X-ray microprobe for environmental and materials sciences
journal, April 2004
- Marcus, Matthew A.; MacDowell, Alastair A.; Celestre, Richard
- Journal of Synchrotron Radiation, Vol. 11, Issue 3
Metal–organic framework-based separator for lithium–sulfur batteries
journal, June 2016
- Bai, Songyan; Liu, Xizheng; Zhu, Kai
- Nature Energy, Vol. 1, Issue 7
Introducing ion-transport-regulating nanochannels to lithium-sulfur batteries
journal, March 2017
- Pan, Yuede; Zhou, Yahong; Zhao, Qing
- Nano Energy, Vol. 33
Characterization of Polysulfide Radicals Present in an Ether-Based Electrolyte of a Lithium-Sulfur Battery During Initial Discharge Using In Situ X-Ray Absorption Spectroscopy Experiments and First-Principles Calculations
journal, June 2015
- Wujcik, Kevin H.; Pascal, Tod A.; Pemmaraju, C. D.
- Advanced Energy Materials, Vol. 5, Issue 16
Exploiting a robust biopolymer network binder for an ultrahigh-areal-capacity Li–S battery
journal, January 2017
- Liu, Jie; Galpaya, Dilini G. D.; Yan, Lijing
- Energy & Environmental Science, Vol. 10, Issue 3
Binder Based on Polyelectrolyte for High Capacity Density Lithium/Sulfur Battery
journal, January 2012
- Zhang, Sheng S.
- Journal of The Electrochemical Society, Vol. 159, Issue 8
A highly ordered nanostructured carbon–sulphur cathode for lithium–sulphur batteries
journal, May 2009
- Ji, Xiulei; Lee, Kyu Tae; Nazar, Linda F.
- Nature Materials, Vol. 8, Issue 6, p. 500-506
Redox-Active Supramolecular Polymer Binders for Lithium–Sulfur Batteries That Adapt Their Transport Properties in Operando
journal, October 2016
- Frischmann, Peter D.; Hwa, Yoon; Cairns, Elton J.
- Chemistry of Materials, Vol. 28, Issue 20
A Family of Highly Ordered Mesoporous Polymer Resin and Carbon Structures from Organic−Organic Self-Assembly
journal, September 2006
- Meng, Yan; Gu, Dong; Zhang, Fuqiang
- Chemistry of Materials, Vol. 18, Issue 18
Sparingly Solvating Electrolytes for High Energy Density Lithium–Sulfur Batteries
journal, August 2016
- Cheng, Lei; Curtiss, Larry A.; Zavadil, Kevin R.
- ACS Energy Letters, Vol. 1, Issue 3
Synthesis of three-dimensionally interconnected sulfur-rich polymers for cathode materials of high-rate lithium–sulfur batteries
journal, June 2015
- Kim, Hoon; Lee, Joungphil; Ahn, Hyungmin
- Nature Communications, Vol. 6, Issue 1
A new class of Solvent-in-Salt electrolyte for high-energy rechargeable metallic lithium batteries
journal, February 2013
- Suo, Liumin; Hu, Yong-Sheng; Li, Hong
- Nature Communications, Vol. 4, Issue 1
The synergetic effect of lithium polysulfide and lithium nitrate to prevent lithium dendrite growth
journal, June 2015
- Li, Weiyang; Yao, Hongbin; Yan, Kai
- Nature Communications, Vol. 6, Issue 1
Surface-enhanced redox chemistry of polysulphides on a metallic and polar host for lithium-sulphur batteries
journal, August 2014
- Pang, Quan; Kundu, Dipan; Cuisinier, Marine
- Nature Communications, Vol. 5, Issue 1
Works referencing / citing this record:
Heterogeneous/Homogeneous Mediators for High-Energy-Density Lithium-Sulfur Batteries: Progress and Prospects
journal, June 2018
- Zhang, Ze-Wen; Peng, Hong-Jie; Zhao, Meng
- Advanced Functional Materials, Vol. 28, Issue 38
Current Status and Future Prospects of Metal–Sulfur Batteries
journal, May 2019
- Chung, Sheng‐Heng; Manthiram, Arumugam
- Advanced Materials, Vol. 31, Issue 27
Highly stable lithium metal battery with an applied three-dimensional mesh structure interlayer
journal, January 2018
- Kim, Hyunjin; Gong, Yong Jun; Yoo, Jeeyoung
- Journal of Materials Chemistry A, Vol. 6, Issue 32
A Review of Functional Binders in Lithium-Sulfur Batteries
journal, October 2018
- Yuan, Hong; Huang, Jia-Qi; Peng, Hong-Jie
- Advanced Energy Materials, Vol. 8, Issue 31
Rational Design of Binders for Stable Li‐S and Na‐S Batteries
journal, December 2019
- Guo, Qianyi; Zheng, Zijian
- Advanced Functional Materials, Vol. 30, Issue 6
High performance potassium–sulfur batteries based on a sulfurized polyacrylonitrile cathode and polyacrylic acid binder
journal, January 2018
- Hwang, Jang-Yeon; Kim, Hee Min; Sun, Yang-Kook
- Journal of Materials Chemistry A, Vol. 6, Issue 30
Ionic Liquids and their Polymers in Lithium‐Sulfur Batteries
journal, January 2019
- Josef, Elinor; Yan, Yajing; Stan, Marian Cristian
- Israel Journal of Chemistry, Vol. 59, Issue 9
Metal Coated Polypropylene Separator with Enhanced Surface Wettability for High Capacity Lithium Metal Batteries
journal, November 2019
- Din, Mir Mehraj Ud; Murugan, Ramaswamy
- Scientific Reports, Vol. 9, Issue 1
Stabilization of Li–S batteries with a lean electrolyte via ion-exchange trapping of lithium polysulfides using a cationic, polybenzimidazolium binder
journal, January 2020
- Pham, Chuyen Van; Liu, Lili; Britton, Benjamin
- Sustainable Energy & Fuels, Vol. 4, Issue 3
Free-Standing Sulfur and Graphitic Porous Carbon Nanofibers Composite Cathode for High Electrochemical Performance of Lithium–Sulfur Batteries
journal, January 2018
- Zhang, Yaoxuan; Wang, Panpan; Tan, Hua
- Journal of The Electrochemical Society, Vol. 165, Issue 5
Bioinspired Binders Actively Controlling Ion Migration and Accommodating Volume Change in High Sulfur Loading Lithium–Sulfur Batteries
journal, November 2019
- Jin, Biyu; Yang, Lifeng; Zhang, Jiawen
- Advanced Energy Materials, Vol. 9, Issue 48
An adaptive and stable bio-electrolyte for rechargeable Zn-ion batteries
journal, January 2018
- Zhang, Silan; Yu, Nengsheng; Zeng, Sha
- Journal of Materials Chemistry A, Vol. 6, Issue 26
Housing Sulfur in Polymer Composite Frameworks for Li–S Batteries
journal, February 2019
- Hencz, Luke; Chen, Hao; Ling, Han Yeu
- Nano-Micro Letters, Vol. 11, Issue 1
PIM-1-based carbon–sulfur composites for sodium–sulfur batteries that operate without the shuttle effect
journal, January 2020
- Jeon, Jun Woo; Kim, Dong-Min; Lee, Jinyoung
- Journal of Materials Chemistry A, Vol. 8, Issue 7
Interfacial active fluorine site-induced electron transfer on TiO 2 (001) facets to enhance polysulfide redox reactions for better liquid Li 2 S 6 -Based lithium–sulfur batteries
journal, January 2019
- Zha, Chenyang; Gu, Xiuquan; Wu, Donghai
- Journal of Materials Chemistry A, Vol. 7, Issue 11
Structural Design of Lithium–Sulfur Batteries: From Fundamental Research to Practical Application
journal, June 2018
- Yang, Xiaofei; Li, Xia; Adair, Keegan
- Electrochemical Energy Reviews, Vol. 1, Issue 3
Porphyrin-Derived Graphene-Based Nanosheets Enabling Strong Polysulfide Chemisorption and Rapid Kinetics in Lithium-Sulfur Batteries
journal, April 2018
- Kong, Long; Li, Bo-Quan; Peng, Hong-Jie
- Advanced Energy Materials, Vol. 8, Issue 20
High Stable Sulfur Cathode with Self‐Healable and Physical Confining Polydimethylsiloxane Interlayer
journal, October 2019
- Cui, Ximing; Pan, Qinmin
- ChemElectroChem, Vol. 6, Issue 22
Recent Advances in Applying Vulcanization/Inverse Vulcanization Methods to Achieve High-Performance Sulfur-Containing Polymer Cathode Materials for Li-S Batteries
journal, August 2018
- Zhao, Fulai; Li, Yu; Feng, Wei
- Small Methods, Vol. 2, Issue 11
Natural Vermiculite Enables High‐Performance in Lithium–Sulfur Batteries via Electrical Double Layer Effects
journal, May 2019
- Wu, Feixiang; Lv, Haifeng; Chen, Shuangqiang
- Advanced Functional Materials
Research Progress of the Solid State Lithium-Sulfur Batteries
journal, October 2019
- Wang, HangChao; Cao, Xin; Liu, Wen
- Frontiers in Energy Research, Vol. 7
Figures / Tables found in this record:

 Search WorldCat to find libraries that may hold this journal
Search WorldCat to find libraries that may hold this journal