Electric Field Effects on the Intermolecular Interactions in Water Whiskers: Insight from Structures, Energetics, and Properties
Abstract
Modulation of intermolecular interactions in response to external electric fields could be fundamental to the formation of unusual forms of water, such as water whiskers. However, a detailed understanding of the nature of intermolecular interactions in such systems is lacking. In this study, we present novel theoretical results based on electron correlation calculations regarding the nature of H-bonds in water whiskers, which is revealed by studying their evolution under external electric fields with various field strengths. We find that the water whiskers consisting of 2-7 water molecules all have a chain-length dependent critical electric field. Under the critical electric field, the most compact chain structures are obtained, featuring very strong H-bonds, herein referred to as covalent H-bonds. In the case of a water dimer whisker, the bond length of the novel covalent H-bond shortens by 25%, the covalent bond order increases by 9 times, and accordingly the H-bond energy is strengthened by 5 times compared to the normal H-bond in a (H2O)2 cluster. Below the critical electric field, it is observed that with increasing field strength, H-bonding orbitals display gradual evolutions in the orbital energy, orbital ordering, and orbital nature (i.e., from typical -style orbital to unusual -style double H-bondingmore »
- Authors:
-
- Jilin Univ., Changchun (China)
- South China Normal University, Guangzhou (China)
- Oak Ridge National Lab. (ORNL), Oak Ridge, TN (United States)
- Publication Date:
- Research Org.:
- Oak Ridge National Laboratory (ORNL), Oak Ridge, TN (United States). Center for Nanophase Materials Sciences (CNMS)
- Sponsoring Org.:
- USDOE Office of Science (SC)
- OSTI Identifier:
- 1265297
- Grant/Contract Number:
- AC05-00OR22725
- Resource Type:
- Accepted Manuscript
- Journal Name:
- Journal of Physical Chemistry. A, Molecules, Spectroscopy, Kinetics, Environment, and General Theory
- Additional Journal Information:
- Journal Volume: 119; Journal Issue: 10; Journal ID: ISSN 1089-5639
- Publisher:
- American Chemical Society
- Country of Publication:
- United States
- Language:
- English
- Subject:
- 37 INORGANIC, ORGANIC, PHYSICAL, AND ANALYTICAL CHEMISTRY
Citation Formats
Bai, Yang, He, Hui-Min, Li, Ying, Zhou, Zhong-Jun, Wang, Jia-Jun, Wu, Di, Chen, Wei, Gu, Feng-Long, Sumpter, Bobby G., and Huang, Jingsong. Electric Field Effects on the Intermolecular Interactions in Water Whiskers: Insight from Structures, Energetics, and Properties. United States: N. p., 2015.
Web. doi:10.1021/jp511460c.
Bai, Yang, He, Hui-Min, Li, Ying, Zhou, Zhong-Jun, Wang, Jia-Jun, Wu, Di, Chen, Wei, Gu, Feng-Long, Sumpter, Bobby G., & Huang, Jingsong. Electric Field Effects on the Intermolecular Interactions in Water Whiskers: Insight from Structures, Energetics, and Properties. United States. https://doi.org/10.1021/jp511460c
Bai, Yang, He, Hui-Min, Li, Ying, Zhou, Zhong-Jun, Wang, Jia-Jun, Wu, Di, Chen, Wei, Gu, Feng-Long, Sumpter, Bobby G., and Huang, Jingsong. Thu .
"Electric Field Effects on the Intermolecular Interactions in Water Whiskers: Insight from Structures, Energetics, and Properties". United States. https://doi.org/10.1021/jp511460c. https://www.osti.gov/servlets/purl/1265297.
@article{osti_1265297,
title = {Electric Field Effects on the Intermolecular Interactions in Water Whiskers: Insight from Structures, Energetics, and Properties},
author = {Bai, Yang and He, Hui-Min and Li, Ying and Zhou, Zhong-Jun and Wang, Jia-Jun and Wu, Di and Chen, Wei and Gu, Feng-Long and Sumpter, Bobby G. and Huang, Jingsong},
abstractNote = {Modulation of intermolecular interactions in response to external electric fields could be fundamental to the formation of unusual forms of water, such as water whiskers. However, a detailed understanding of the nature of intermolecular interactions in such systems is lacking. In this study, we present novel theoretical results based on electron correlation calculations regarding the nature of H-bonds in water whiskers, which is revealed by studying their evolution under external electric fields with various field strengths. We find that the water whiskers consisting of 2-7 water molecules all have a chain-length dependent critical electric field. Under the critical electric field, the most compact chain structures are obtained, featuring very strong H-bonds, herein referred to as covalent H-bonds. In the case of a water dimer whisker, the bond length of the novel covalent H-bond shortens by 25%, the covalent bond order increases by 9 times, and accordingly the H-bond energy is strengthened by 5 times compared to the normal H-bond in a (H2O)2 cluster. Below the critical electric field, it is observed that with increasing field strength, H-bonding orbitals display gradual evolutions in the orbital energy, orbital ordering, and orbital nature (i.e., from typical -style orbital to unusual -style double H-bonding orbital). We also show that beyond the critical electric field, a single water whisker may disintegrate to form a loosely bound zwitterionic chain due to a relay-style proton transfer, whereas two water whiskers may undergo intermolecular cross-linking to form a quasi-two-dimensional water network. In conclusion, these results help shed new insight on the effects of electric fields on water whisker formation.},
doi = {10.1021/jp511460c},
journal = {Journal of Physical Chemistry. A, Molecules, Spectroscopy, Kinetics, Environment, and General Theory},
number = 10,
volume = 119,
place = {United States},
year = {Thu Feb 19 00:00:00 EST 2015},
month = {Thu Feb 19 00:00:00 EST 2015}
}
Web of Science
Figures / Tables:
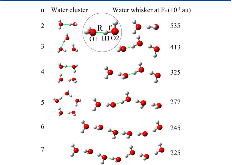 Figure 1: Linear chain structures of water whiskers with 2−7 water molecules, under respective critical electric fields (Fc). The Fc decreases with increasing chain length. The linear chain (n = 2) and cyclic structures (n = 3−5) of typical (H2O)n water clusters are also shown for comparison. All structures weremore »
Figure 1: Linear chain structures of water whiskers with 2−7 water molecules, under respective critical electric fields (Fc). The Fc decreases with increasing chain length. The linear chain (n = 2) and cyclic structures (n = 3−5) of typical (H2O)n water clusters are also shown for comparison. All structures weremore »
Works referenced in this record:
A molecular perspective of water at metal interfaces
journal, July 2012
- Carrasco, Javier; Hodgson, Andrew; Michaelides, Angelos
- Nature Materials, Vol. 11, Issue 8
Controversy in Chemistry: How Do You Prove a Negative??The Cases of Phlogiston and Cold Fusion
journal, March 2005
- Labinger, Jay A.; Weininger, Stephen J.
- Angewandte Chemie International Edition, Vol. 44, Issue 13
Does the Hydrated Electron Occupy a Cavity?
journal, July 2010
- Larsen, R. E.; Glover, W. J.; Schwartz, B. J.
- Science, Vol. 329, Issue 5987
A direct MP2 gradient method
journal, February 1990
- Frisch, Michael J.; Head-Gordon, Martin; Pople, John A.
- Chemical Physics Letters, Vol. 166, Issue 3
Water whiskers in high electric fields
journal, January 2011
- Karahka, M.; Kreuzer, H. J.
- Physical Chemistry Chemical Physics, Vol. 13, Issue 23
Energy landscapes for water clusters in a uniform electric field
journal, February 2007
- James, Tim; Wales, David J.; Hernández Rojas, Javier
- The Journal of Chemical Physics, Vol. 126, Issue 5
Mechanism of Fast Proton Transport along One-Dimensional Water Chains Confined in Carbon Nanotubes
journal, August 2010
- Cao, Zhen; Peng, Yuxing; Yan, Tianying
- Journal of the American Chemical Society, Vol. 132, Issue 33
"Polywater": Evidence from Electron Spectroscopy for Chemical Analysis (ESCA) of a Complex Salt Mixture
journal, January 1971
- Davis, R. E.; Rousseau, D. L.; Board, R. D.
- Science, Vol. 171, Issue 3967
Unusual Hydrogen Bonding in Water-Filled Carbon Nanotubes
journal, September 2006
- Byl, Oleg; Liu, Jin-Chen; Wang, Yang
- Journal of the American Chemical Society, Vol. 128, Issue 37
Hydrogen bonding between the water molecule and the hydroxyl radical (H 2 O⋅HO): The global minimum
journal, June 1993
- Xie, Yaoming; Schaefer, Henry F.
- The Journal of Chemical Physics, Vol. 98, Issue 11
Water structural transformation at molecular hydrophobic interfaces
journal, November 2012
- Davis, Joel G.; Gierszal, Kamil P.; Wang, Ping
- Nature, Vol. 491, Issue 7425
Ultrafast vibrational energy transfer at the water/air interface revealed by two-dimensional surface vibrational spectroscopy
journal, October 2011
- Zhang, Zhen; Piatkowski, Lukasz; Bakker, Huib J.
- Nature Chemistry, Vol. 3, Issue 11
"Polywater" and Sweat: Similarities between the Infrared Spectra
journal, January 1971
- Rousseau, D. L.
- Science, Vol. 171, Issue 3967
Interaction of water with field emitter tips
journal, January 1995
- Ciszewski, Antoni; Błaszczyszyn, Ryszard
- Progress in Surface Science, Vol. 48, Issue 1-4
Anomalously Soft Dynamics of Water in a Nanotube: A Revelation of Nanoscale Confinement
journal, July 2004
- Kolesnikov, Alexander I.; Zanotti, Jean-Marc; Loong, Chun-Keung
- Physical Review Letters, Vol. 93, Issue 3
Massenspektrometrische Untersuchung der Feldionisation von Wasserdampf an Spitzen aus Wolfram, Platin und Iridium
journal, March 1964
- Schmidt, W. A.
- Zeitschrift für Naturforschung A, Vol. 19, Issue 3
Electric field effects on water clusters (n=3–5): Systematic ab initio study of structures, energetics, and transition states
journal, March 2006
- Choi, Young Cheol; Pak, Chaeho; Kim, Kwang S.
- The Journal of Chemical Physics, Vol. 124, Issue 9
Avoiding the integral storage bottleneck in LCAO calculations of electron correlation
journal, January 1989
- Sæbø, Svein; Almlöf, Jan
- Chemical Physics Letters, Vol. 154, Issue 1
Analytic MP2 frequencies without fifth-order storage. Theory and application to bifurcated hydrogen bonds in the water hexamer
journal, March 1994
- Head-Gordon, Martin; Head-Gordon, Teresa
- Chemical Physics Letters, Vol. 220, Issue 1-2
Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen
journal, January 1989
- Dunning, Thom H.
- The Journal of Chemical Physics, Vol. 90, Issue 2
Conduction and Electrostriction of Polymers Induced by High Electric Fields
journal, December 2010
- Karahka, Markus; Kreuzer, Hans Jürgen
- Polymers, Vol. 3, Issue 1
Hydrogen-Bond Stereochemistry and "Anomalous Water"
journal, April 1971
- Kamb, B.
- Science, Vol. 172, Issue 3980
Polywater
journal, June 1969
- Lippincott, E. R.; Stromberg, R. R.; Grant, W. H.
- Science, Vol. 164, Issue 3887
Field-Induced Structural Changes in Adsorbed Layers of Polar Molecules Studied by Photon-Stimulated Desorption
journal, November 1988
- Jaenicke, S.; Ciszewski, A.; DÖSselmann, J.
- Le Journal de Physique Colloques, Vol. 49, Issue C6
Water in Nonpolar Confinement: From Nanotubes to Proteins and Beyond
journal, May 2008
- Rasaiah, Jayendran C.; Garde, Shekhar; Hummer, Gerhard
- Annual Review of Physical Chemistry, Vol. 59, Issue 1
Water clusters (H2O)n, n=6–8, in external electric fields
journal, January 2008
- Rai, Dhurba; Kulkarni, Anant D.; Gejji, Shridhar P.
- The Journal of Chemical Physics, Vol. 128, Issue 3
DFT Investigation of Alkyl Sulfate Surfactant Adsorption at the Air-Water Interface
journal, January 2009
- Chen, Mei-Ling; Wang, Zheng-Wu; Tao, Fu-Ming
- Journal of Dispersion Science and Technology, Vol. 30, Issue 2
Electron-Driven Acid-Base Chemistry: Proton Transfer from Hydrogen Chloride to Ammonia
journal, February 2008
- Eustis, Soren N.; Radisic, Dunja; Bowen, Kit H.
- Science, Vol. 319, Issue 5865
Water Confined in Nanotubes and between Graphene Sheets: A First Principle Study
journal, January 2008
- Cicero, Giancarlo; Grossman, Jeffrey C.; Schwegler, Eric
- Journal of the American Chemical Society, Vol. 130, Issue 6
Note on an Approximation Treatment for Many-Electron Systems
journal, October 1934
- Møller, Chr.; Plesset, M. S.
- Physical Review, Vol. 46, Issue 7
The interaction of synchrotron light with water adsorbed on a Ag-field emitter in the presence of a high electric field
journal, January 1992
- Dirks, J.
- Journal of Vacuum Science & Technology B: Microelectronics and Nanometer Structures, Vol. 10, Issue 1
Field Ionization of Water
journal, March 1969
- Anway, Allen R.
- The Journal of Chemical Physics, Vol. 50, Issue 5
Semi-direct algorithms for the MP2 energy and gradient
journal, February 1990
- Frisch, Michael J.; Head-Gordon, Martin; Pople, John A.
- Chemical Physics Letters, Vol. 166, Issue 3
Field-Assisted Photodesorption of ions from Metal and Semiconductor Surfaces
journal, November 1986
- Jaenicke, S.; Ciszewski, A.; Drachsel, W.
- Le Journal de Physique Colloques, Vol. 47, Issue C7
Electric Field-Driven Acid−Base Chemistry: Proton Transfer from Acid (HCl) to Base (NH 3 /H 2 O)
journal, March 2011
- Zhou, Zhong-Jun; Li, Xiao-Ping; Liu, Zhen-Bo
- The Journal of Physical Chemistry A, Vol. 115, Issue 8
Massenspektrometrische Untersuchungen über Ionen-Molekülreaktionen und über die Assoziation des Wassers mit Hilfe einer Feldemissions-Ionenquelle
journal, September 1960
- Beckey, H. D.
- Zeitschrift für Naturforschung A, Vol. 15, Issue 9
Works referencing / citing this record:
Energetic aminated-azole assemblies from intramolecular and intermolecular N–H⋯O and N–H⋯N hydrogen bonds
journal, January 2016
- He, Chunlin; Yin, Ping; Mitchell, Lauren A.
- Chemical Communications, Vol. 52, Issue 52
One-dimensional water nanowires induced by electric fields
journal, January 2019
- Zhao, Wan; Huang, Haishen; Bi, Qingling
- Physical Chemistry Chemical Physics, Vol. 21, Issue 35
Some measures for making a traditional halogen bond be chlorine-shared or ion-pair one in FCl•NH 3 complex
journal, November 2016
- Xu, Huili; Cheng, Jianbo; Li, Qingzhong
- Molecular Physics, Vol. 114, Issue 24
Some measures for making a traditional halogen bond be chlorine-shared or ion-pair one in FCl•NH3 complex
text, January 2016
- Xu, Huili; Cheng, Jianbo; Li, Qingzhong
- Taylor & Francis
Some measures for making a traditional halogen bond be chlorine-shared or ion-pair one in FCl•NH3 complex
text, January 2016
- Xu, Huili; Cheng, Jianbo; Li, Qingzhong
- Taylor & Francis

 Search WorldCat to find libraries that may hold this journal
Search WorldCat to find libraries that may hold this journal