Synthesis of Cycloprodigiosin Identifies the Natural Isolate as a Scalemic Mixture
Abstract
We prepared the enantiomers of the natural product cycloprodigiosin using an expedient five-step synthetic sequence that takes advantage of a Schöllkopf–Barton–Zard (SBZ) pyrrole annulation with a chiral isocyanoacetate and a nitrocyclohexene derivative. Using chiral HPLC and X-ray crystallographic analyses of the synthetically prepared material and natural isolate (isolated from the marine bacterium Pseudoalteromonas rubra), naturally occurring cycloprodigiosin was determined to be a scalemic mixture occurring in an enantiomeric ratio of 83:17 (R)/(S) at C4'.
- Authors:
- Publication Date:
- Research Org.:
- Lawrence Berkeley National Laboratory (LBNL), Berkeley, CA (United States)
- Sponsoring Org.:
- USDOE Office of Science (SC), Biological and Environmental Research (BER)
- OSTI Identifier:
- 1257348
- Grant/Contract Number:
- AC02-05CH11231; S10-RR027172; 1341894
- Resource Type:
- Accepted Manuscript
- Journal Name:
- Organic Letters
- Additional Journal Information:
- Journal Volume: 17; Journal Issue: 14; Journal ID: ISSN 1523-7060
- Publisher:
- American Chemical Society
- Country of Publication:
- United States
- Language:
- English
- Subject:
- 37 INORGANIC, ORGANIC, PHYSICAL, AND ANALYTICAL CHEMISTRY
Citation Formats
Johnson, Rebecca E., de Rond, Tristan, Lindsay, Vincent N. G., Keasling, Jay D., and Sarpong, Richmond. Synthesis of Cycloprodigiosin Identifies the Natural Isolate as a Scalemic Mixture. United States: N. p., 2015.
Web. doi:10.1021/acs.orglett.5b01527.
Johnson, Rebecca E., de Rond, Tristan, Lindsay, Vincent N. G., Keasling, Jay D., & Sarpong, Richmond. Synthesis of Cycloprodigiosin Identifies the Natural Isolate as a Scalemic Mixture. United States. https://doi.org/10.1021/acs.orglett.5b01527
Johnson, Rebecca E., de Rond, Tristan, Lindsay, Vincent N. G., Keasling, Jay D., and Sarpong, Richmond. Fri .
"Synthesis of Cycloprodigiosin Identifies the Natural Isolate as a Scalemic Mixture". United States. https://doi.org/10.1021/acs.orglett.5b01527. https://www.osti.gov/servlets/purl/1257348.
@article{osti_1257348,
title = {Synthesis of Cycloprodigiosin Identifies the Natural Isolate as a Scalemic Mixture},
author = {Johnson, Rebecca E. and de Rond, Tristan and Lindsay, Vincent N. G. and Keasling, Jay D. and Sarpong, Richmond},
abstractNote = {We prepared the enantiomers of the natural product cycloprodigiosin using an expedient five-step synthetic sequence that takes advantage of a Schöllkopf–Barton–Zard (SBZ) pyrrole annulation with a chiral isocyanoacetate and a nitrocyclohexene derivative. Using chiral HPLC and X-ray crystallographic analyses of the synthetically prepared material and natural isolate (isolated from the marine bacterium Pseudoalteromonas rubra), naturally occurring cycloprodigiosin was determined to be a scalemic mixture occurring in an enantiomeric ratio of 83:17 (R)/(S) at C4'.},
doi = {10.1021/acs.orglett.5b01527},
journal = {Organic Letters},
number = 14,
volume = 17,
place = {United States},
year = {Fri Jul 17 00:00:00 EDT 2015},
month = {Fri Jul 17 00:00:00 EDT 2015}
}
Free Publicly Available Full Text
Publisher's Version of Record
Other availability
Cited by: 12 works
Citation information provided by
Web of Science
Web of Science
Figures / Tables:
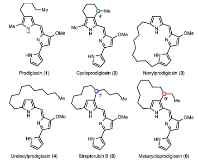 Figure 1.: Representative members in the prodigiosin family of alkaloids.
Figure 1.: Representative members in the prodigiosin family of alkaloids.
All figures and tables
(6 total)
Save to My Library
You must Sign In or Create an Account in order to save documents to your library.
Works referenced in this record:
Cycloprodigiosin hydrochloride, a new H+/Cl? symporter, induces apoptosis in human and rat hepatocellular cancer cell linesin vitro and inhibits the growth of hepatocellular carcinoma xenografts in nude mice
journal, October 1999
- Yamamoto, Chizuko; Takemoto, Hiroto; Kuno, Kenji
- Hepatology, Vol. 30, Issue 4
Induction of apoptosis of activated murine splenic T cells by cycloprodigiosin hydrochloride, a novel immunosuppressant
journal, January 2000
- Azuma, Takayoshi; Watanabe, Naoko; Yagisawa, Hitoshi
- Immunopharmacology, Vol. 46, Issue 1
Cycloprodigiosin Hydrochloride Obtained from Pseudoalteromonas denitrificans Is a Potent Antimalarial Agent.
journal, January 1999
- Kim, Hye-Sook; Hayashi, Mitsuko; Shibata, Yasuharu
- Biological and Pharmaceutical Bulletin, Vol. 22, Issue 5
Chemistry and Biology of Roseophilin and the Prodigiosin Alkaloids: A Survey of the Last 2500 Years
journal, August 2003
- Fürstner, Alois
- Angewandte Chemie International Edition, Vol. 42, Issue 31
Prodigiosin alkaloids: recent advancements in total synthesis and their biological potential
journal, January 2015
- Nisha, Nisha; Kumar, Kewal; Kumar, Vipan
- RSC Advances, Vol. 5, Issue 15
The biosynthesis and regulation of bacterial prodiginines
journal, December 2006
- Williamson, Neil R.; Fineran, Peter C.; Leeper, Finian J.
- Nature Reviews Microbiology, Vol. 4, Issue 12
Influence of the A -Ring on the Proton Affinity and Anticancer Properties of the Prodigiosins
journal, April 2002
- Melvin, Matt S.; Tomlinson, John T.; Park, Gyungse
- Chemical Research in Toxicology, Vol. 15, Issue 5
Prodigiosins uncouple lysosomal vacuolar-type ATPase through promotion of H+/Cl− symport
journal, September 1998
- Ohkuma, Shoji; Sato, Tomohiko; Okamoto, Masayuki
- Biochemical Journal, Vol. 334, Issue 3
Prodigiosin is a chloride carrier that can function as an anion exchanger
journal, January 2005
- Seganish, Jennifer L.; Davis, Jeffery T.
- Chemical Communications, Issue 46
Molecular Interactions of Prodiginines with the BH3 Domain of Anti-Apoptotic Bcl-2 Family Members
journal, February 2013
- Hosseini, Ali; Espona-Fiedler, Margarita; Soto-Cerrato, Vanessa
- PLoS ONE, Vol. 8, Issue 2
Elimination of Butylcycloheptylprodigiosin as a Known Natural Product Inspired by an Evolutionary Hypothesis for Cyclic Prodigiosin Biosynthesis
journal, September 2013
- Jones, Brian T.; Hu, Dennis X.; Savoie, Brett M.
- Journal of Natural Products, Vol. 76, Issue 10
Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis
journal, November 2007
- Nguyen, M.; Marcellus, R. C.; Roulston, A.
- Proceedings of the National Academy of Sciences, Vol. 104, Issue 49
Stereochemical Elucidation of Streptorubin B
journal, February 2011
- Haynes, Stuart W.; Sydor, Paulina K.; Corre, Christophe
- Journal of the American Chemical Society, Vol. 133, Issue 6
Regio- and stereodivergent antibiotic oxidative carbocyclizations catalysed by Rieske oxygenase-like enzymes
journal, April 2011
- Sydor, Paulina K.; Barry, Sarah M.; Odulate, Olanipekun M.
- Nature Chemistry, Vol. 3, Issue 5
C–H activation is a Reiske business
journal, April 2011
- Bruner, Steven D.
- Nature Chemistry, Vol. 3, Issue 5
The biosynthesis of metacycloprodigiosin and undecylprodigiosin
journal, January 1974
- Wasserman, H. H.; Shaw, C. K.; Sykes, R. J.
- Tetrahedron Letters, Vol. 15, Issue 33
Cycloprodigiosin from beneckea gazogenes
journal, January 1983
- Gerber, Nancy N.
- Tetrahedron Letters, Vol. 24, Issue 27
A revised structure for cycloprodigiosin
journal, January 1983
- Lattasch, Hartmut; Thomson, Ronald H.
- Tetrahedron Letters, Vol. 24, Issue 26
The synthesis of (±)-cycloprodigiosin
journal, January 1984
- Wasserman, Harry H.; Fukuyama, James M.
- Tetrahedron Letters, Vol. 25, Issue 13
Enantioselective Total Synthesis and Confirmation of the Absolute and Relative Stereochemistry of Streptorubin B
journal, February 2011
- Hu, Dennis X.; Clift, Michael D.; Lazarski, Kiel E.
- Journal of the American Chemical Society, Vol. 133, Issue 6
Application of In Situ-Generated Rh-Bound Trimethylenemethane Variants to the Synthesis of 3,4-Fused Pyrroles
journal, March 2013
- Schultz, Erica E.; Sarpong, Richmond
- Journal of the American Chemical Society, Vol. 135, Issue 12
A new synthesis of pyrroles from nitroalkenes
journal, January 1985
- Barton, Derek H. R.; Zard, Samir Z.
- Journal of the Chemical Society, Chemical Communications, Issue 16
A new synthesis of conjugated nitro cyclo olefins, unusually versatile synthetic intermediates
journal, September 1978
- Corey, E. J.; Estreicher, Herbert
- Journal of the American Chemical Society, Vol. 100, Issue 19
C–H Methylation of Heteroarenes Inspired by Radical SAM Methyl Transferase
journal, March 2014
- Gui, Jinghan; Zhou, Qianghui; Pan, Chung-Mao
- Journal of the American Chemical Society, Vol. 136, Issue 13
Manganese(III) Complexes of Bis(hydroxyphenyl)dipyrromethenes Are Potent Orally Active Peroxynitrite Scavengers
journal, March 2011
- Rausaria, Smita; Kamadulski, Andrew; Rath, Nigam P.
- Journal of the American Chemical Society, Vol. 133, Issue 12
Two-step synthesis of the bipyrrole precursor of prodigiosins
journal, April 2006
- Dairi, Kenza; Tripathy, Sasmita; Attardo, Giorgio
- Tetrahedron Letters, Vol. 47, Issue 15
Facile synthesis of a chiral auxiliary bearing the isocyanide group
journal, January 2001
- Ilankumaran, Palanichamy; Kisanga, Philip; Verkade, John G.
- Heteroatom Chemistry, Vol. 12, Issue 7
Colour Change of Prodigiosin
journal, October 1968
- Hearn, Walter R.; Medina-Castro, Jorge; Elson, Michael K.
- Nature, Vol. 220, Issue 5163
Stereochemical Elucidation of Streptorubin B
journal, February 2011
- Haynes, Stuart W.; Sydor, Paulina K.; Corre, Christophe
- Journal of the American Chemical Society, Vol. 133, Issue 6
Enantioselective Total Synthesis and Confirmation of the Absolute and Relative Stereochemistry of Streptorubin B
journal, February 2011
- Hu, Dennis X.; Clift, Michael D.; Lazarski, Kiel E.
- Journal of the American Chemical Society, Vol. 133, Issue 6
Manganese(III) Complexes of Bis(hydroxyphenyl)dipyrromethenes Are Potent Orally Active Peroxynitrite Scavengers
journal, March 2011
- Rausaria, Smita; Kamadulski, Andrew; Rath, Nigam P.
- Journal of the American Chemical Society, Vol. 133, Issue 12
Application of In Situ-Generated Rh-Bound Trimethylenemethane Variants to the Synthesis of 3,4-Fused Pyrroles
journal, March 2013
- Schultz, Erica E.; Sarpong, Richmond
- Journal of the American Chemical Society, Vol. 135, Issue 12
C–H Methylation of Heteroarenes Inspired by Radical SAM Methyl Transferase
journal, March 2014
- Gui, Jinghan; Zhou, Qianghui; Pan, Chung-Mao
- Journal of the American Chemical Society, Vol. 136, Issue 13
Elimination of Butylcycloheptylprodigiosin as a Known Natural Product Inspired by an Evolutionary Hypothesis for Cyclic Prodigiosin Biosynthesis
journal, September 2013
- Jones, Brian T.; Hu, Dennis X.; Savoie, Brett M.
- Journal of Natural Products, Vol. 76, Issue 10
Colour Change of Prodigiosin
journal, October 1968
- Hearn, Walter R.; Medina-Castro, Jorge; Elson, Michael K.
- Nature, Vol. 220, Issue 5163
The biosynthesis and regulation of bacterial prodiginines
journal, December 2006
- Williamson, Neil R.; Fineran, Peter C.; Leeper, Finian J.
- Nature Reviews Microbiology, Vol. 4, Issue 12
Prodigiosins uncouple lysosomal vacuolar-type ATPase through promotion of H+/Cl− symport
journal, September 1998
- Ohkuma, Shoji; Sato, Tomohiko; Okamoto, Masayuki
- Biochemical Journal, Vol. 334, Issue 3
Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis
journal, November 2007
- Nguyen, M.; Marcellus, R. C.; Roulston, A.
- Proceedings of the National Academy of Sciences, Vol. 104, Issue 49
Cycloprodigiosin Hydrochloride Obtained from Pseudoalteromonas denitrificans Is a Potent Antimalarial Agent.
journal, January 1999
- Kim, Hye-Sook; Hayashi, Mitsuko; Shibata, Yasuharu
- Biological and Pharmaceutical Bulletin, Vol. 22, Issue 5
Molecular Interactions of Prodiginines with the BH3 Domain of Anti-Apoptotic Bcl-2 Family Members
journal, February 2013
- Hosseini, Ali; Espona-Fiedler, Margarita; Soto-Cerrato, Vanessa
- PLoS ONE, Vol. 8, Issue 2
Works referencing / citing this record:
Preparation of Cyclic Prodiginines by Mutasynthesis in Pseudomonas putida KT2440
journal, May 2018
- Klein, Andreas Sebastian; Brass, Hannah Ursula Clara; Klebl, David Paul
- ChemBioChem, Vol. 19, Issue 14
Synthesis of Highly Enantio-Enriched Heliespirones A and C by a Diastereoselective Aromatic Claisen Rearrangement
journal, January 2018
- Norcott, Philip; McErlean, Christopher S. P.
- Australian Journal of Chemistry, Vol. 71, Issue 5
Culturing marine bacteria from the genusPseudoalteromonason a cotton scaffold alters secondary metabolite production
journal, October 2018
- Timmermans, Marshall L.; Picott, Katherine J.; Ucciferri, Lorena
- MicrobiologyOpen, Vol. 8, Issue 5
Oxidative cyclization of prodigiosin by an alkylglycerol monooxygenase-like enzyme
journal, September 2017
- de Rond, Tristan; Stow, Parker; Eigl, Ian
- Nature Chemical Biology, Vol. 13, Issue 11
Figures / Tables found in this record:
Figures/Tables have been extracted from DOE-funded journal article accepted manuscripts.

 Search WorldCat to find libraries that may hold this journal
Search WorldCat to find libraries that may hold this journal