The Plasticity of Molecular Interactions Governs Bacterial Microcompartment Shell Assembly
Abstract
Bacterial microcompartments (BMCs) are composed of an enzymatic core encapsulated by a selectively permeable protein shell that enhances catalytic efficiency. Many pathogenic bacteria derive a competitive advantage from their BMC based catabolism, implicating BMCs as promising drug targets. BMC shells are of interest for bioengineering due to their diverse and selective permeability properties and because they self-assemble. A complete understanding of shell composition and organization is a prerequisite for biotechnological applications. Here, we report the cryo-EM structure of a BMC shell at 3.0 32 Å resolution, using an image processing strategy that allowed us to determine the previously uncharacterized structural details of the interactions of the BMC-TS and BMC-TD shell subunits with the surrounding BMC-H subunits in the context of the assembled shell. We found unexpected structural plasticity among these interactions, resulting in distinct shell populations assembled from varying numbers of the BMC-TS and BMC-TD shell subunits. Here, our results show that heterologously produced shells are surprisingly compositonally diverse; we discuss the implications of these findings on shell assembly and function.
- Authors:
- Publication Date:
- Research Org.:
- Michigan State Univ., East Lansing, MI (United States)
- Sponsoring Org.:
- USDOE Office of Science (SC), Basic Energy Sciences (BES); National Institutes of Health, National Institute of Allergy and Infectious Diseases (NIAID)
- OSTI Identifier:
- 1617538
- Alternate Identifier(s):
- OSTI ID: 1671308
- Grant/Contract Number:
- FG02-91ER20021; R01 AI114975-05; AC02-05CH11231
- Resource Type:
- Journal Article: Published Article
- Journal Name:
- Structure
- Additional Journal Information:
- Journal Name: Structure Journal Volume: 27 Journal Issue: 5; Journal ID: ISSN 0969-2126
- Publisher:
- Elsevier
- Country of Publication:
- United Kingdom
- Language:
- English
- Subject:
- 59 BASIC BIOLOGICAL SCIENCES; Bacterial microcompartments; microbiology; metabolosome; modular assembly; bioengineering; structural biology; permeability; cryoelectron microscopy
Citation Formats
Greber, Basil J., Sutter, Markus, and Kerfeld, Cheryl A. The Plasticity of Molecular Interactions Governs Bacterial Microcompartment Shell Assembly. United Kingdom: N. p., 2019.
Web. doi:10.1016/j.str.2019.01.017.
Greber, Basil J., Sutter, Markus, & Kerfeld, Cheryl A. The Plasticity of Molecular Interactions Governs Bacterial Microcompartment Shell Assembly. United Kingdom. https://doi.org/10.1016/j.str.2019.01.017
Greber, Basil J., Sutter, Markus, and Kerfeld, Cheryl A. 2019.
"The Plasticity of Molecular Interactions Governs Bacterial Microcompartment Shell Assembly". United Kingdom. https://doi.org/10.1016/j.str.2019.01.017.
@article{osti_1617538,
title = {The Plasticity of Molecular Interactions Governs Bacterial Microcompartment Shell Assembly},
author = {Greber, Basil J. and Sutter, Markus and Kerfeld, Cheryl A.},
abstractNote = {Bacterial microcompartments (BMCs) are composed of an enzymatic core encapsulated by a selectively permeable protein shell that enhances catalytic efficiency. Many pathogenic bacteria derive a competitive advantage from their BMC based catabolism, implicating BMCs as promising drug targets. BMC shells are of interest for bioengineering due to their diverse and selective permeability properties and because they self-assemble. A complete understanding of shell composition and organization is a prerequisite for biotechnological applications. Here, we report the cryo-EM structure of a BMC shell at 3.0 32 Å resolution, using an image processing strategy that allowed us to determine the previously uncharacterized structural details of the interactions of the BMC-TS and BMC-TD shell subunits with the surrounding BMC-H subunits in the context of the assembled shell. We found unexpected structural plasticity among these interactions, resulting in distinct shell populations assembled from varying numbers of the BMC-TS and BMC-TD shell subunits. Here, our results show that heterologously produced shells are surprisingly compositonally diverse; we discuss the implications of these findings on shell assembly and function.},
doi = {10.1016/j.str.2019.01.017},
url = {https://www.osti.gov/biblio/1617538},
journal = {Structure},
issn = {0969-2126},
number = 5,
volume = 27,
place = {United Kingdom},
year = {Wed May 01 00:00:00 EDT 2019},
month = {Wed May 01 00:00:00 EDT 2019}
}
Web of Science
Figures / Tables:
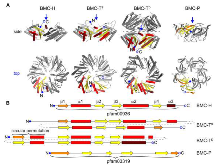 Fig. 1: Component structures of the HO BMC shell. (A) Cartoon representation of structures of the BMC shell proteins found in the HO shell, viewed from the side (top row) and from the top onto the concave side (bottom row; view point indicated by a blue arrow in the sidemore »
Fig. 1: Component structures of the HO BMC shell. (A) Cartoon representation of structures of the BMC shell proteins found in the HO shell, viewed from the side (top row) and from the top onto the concave side (bottom row; view point indicated by a blue arrow in the sidemore »
Works referencing / citing this record:
Engineering the PduT shell protein to modify the permeability of the 1,2-propanediol microcompartment of Salmonella
journal, December 2019
- Chowdhury, Chiranjit; Bobik, Thomas A.
- Microbiology, Vol. 165, Issue 12
Functionalization of Bacterial Microcompartment Shell Proteins With Covalently Attached Heme
journal, January 2020
- Huang, Jingcheng; Ferlez, Bryan H.; Young, Eric J.
- Frontiers in Bioengineering and Biotechnology, Vol. 7
Figures / Tables found in this record: