|
ATHENA , ARTEMIS , HEPHAESTUS : data analysis for X-ray absorption spectroscopy using IFEFFIT
|
journal
|
June 2005 |
|
Infrared refractive index of diamond
|
journal
|
January 1981 |
|
Refractive index calculation using the structural properties of La4Ti9O24 glass
|
journal
|
May 2008 |
|
Density of thin TiO2 films
|
journal
|
June 1996 |
|
Phase relationship in the TiO2–Nd2O3 pseudo-binary system
|
journal
|
January 2013 |
|
Liquid B 2 O 3 up to 1700 K: x-ray diffraction and boroxol ring dissolution
|
journal
|
October 2015 |
|
Continuous Structural Transition in Glass-Forming Molten Titanate BaTi 2 O 5
|
journal
|
November 2016 |
|
Structure of binary K2OTiO2 and Cs2OTiO2 glasses
|
journal
|
October 1989 |
|
Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides
|
journal
|
September 1976 |
|
Study on upconversion luminescence and thermal properties of Ho3+/Yb3+ co-doped La2O3–TiO2–ZrO2 glasses
|
journal
|
July 2016 |
|
Aerodynamic levitator for in situ x-ray structure measurements on high temperature and molten nuclear fuel materials
|
journal
|
July 2016 |
|
Glass formation of rare earth aluminates by containerless processing
|
journal
|
December 2012 |
|
Natural widths of atomic K and L levels, K α X‐ray lines and several K L L Auger lines
|
journal
|
April 1979 |
|
Temperature-Driven Structural Transitions in Molten Sodium Borates Na 2 O–B 2 O 3 : X-ray Diffraction, Thermodynamic Modeling, and Implications for Topological Constraint Theory
|
journal
|
December 2015 |
|
Borate melt structure: Temperature‐dependent B–O bond lengths and coordination numbers from high‐energy X‐ray diffraction
|
journal
|
March 2018 |
|
Comprehensive Structural Study of Glassy and Metastable Crystalline BaTi 2 O 5
|
journal
|
January 2009 |
|
Glass Formation in LaO3/2-TiO2 Binary System by Containerless Processing
|
journal
|
December 2011 |
|
Optical super-resolution by high-index liquid-immersed microspheres
|
journal
|
October 2012 |
|
Laser hearth melt processing of ceramic materials
|
journal
|
February 1996 |
|
Rapid quenching on the binary systems of high temperature oxides
|
journal
|
June 1974 |
|
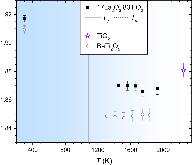
The Density of Titanium(IV) Oxide Liquid
|
journal
|
October 1991 |
|
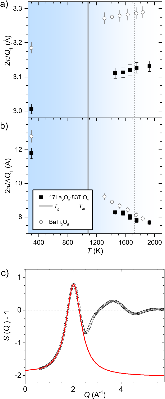
Structure of molten titanium dioxide
|
journal
|
September 2014 |
|
Molten barium titanate: a high-pressure liquid silicate analogue
|
journal
|
March 2019 |
|
Vitreous forsterite (Mg 2 SiO 4 ): Synthesis, structure, and thermochemistry
|
journal
|
July 2001 |
|
Low Cation Coordination in Oxide Melts
|
journal
|
April 2014 |
|
Influence of the Atmosphere on the Oxidation State of the Eu-Ion in a SiAIO(N) Glass and Glass-Ceramic
|
journal
|
January 2000 |
|
Local structural variation with oxygen fugacity in Fe2SiO4+ fayalitic iron silicate melts
|
journal
|
April 2017 |
|
Glass-forming region and high refractive index of TiO 2 -based glasses prepared by containerless processing : Glass-forming region and high refractive index of TiO
|
journal
|
October 2012 |
|
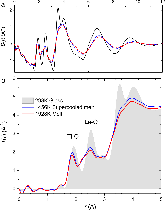
Investigation of TiO2SiO2 glasses by X-ray absorption spectroscopy
|
journal
|
April 1983 |
|
Refractive index dispersion, optical transmittance, and Raman scattering of BaTi2O5 glass
|
journal
|
September 2010 |
|
Measurements of liquid and glass structures using aerodynamic levitation and in-situ high energy x-ray and neutron scattering
|
journal
|
January 2014 |
|
Phase Relations in the System Y2O3-TiO2
|
journal
|
March 1976 |
|
Structure of Na2O�2TiO2 glass
|
journal
|
January 1991 |
|
Phase stability and equilibria in the La2O3–TiO2 system
|
journal
|
July 2000 |
|
Study on the structure of K2O·2TiO2 glass by X-ray radial distribution analysis
|
journal
|
January 1989 |
|
Synthesis of amorphous La 4 Ti 9 O 24 microspheres with high-refractive index via containerless flame-spraying method
|
journal
|
January 2018 |
|
Solidification and thermophysical property studies of barium titanate using electrostatic levitation furnace
|
journal
|
July 2006 |
|
Spectral Absorption Coefficient of Molten Aluminum Oxide from.0.385 to 0.780 mum
|
journal
|
March 1995 |
|
Phase equilibrium relationships in the system Gd2O3-TiO2
|
journal
|
May 1965 |
|
The Structural Role of Lanthanum and Yttrium in Aluminosilicate Glasses: A 27 Al and 17 O MAS NMR Study
|
journal
|
December 1998 |
|
Bright white upconversion luminescence from Er3+/Tm3+/Yb3+-doped titanate-based glasses prepared by aerodynamic levitation method
|
journal
|
October 2017 |
|
Iron K-edge X-ray absorption near-edge structure spectroscopy of aerodynamically levitated silicate melts and glasses
|
journal
|
March 2017 |
|
Density and refractive index of TiO2 films prepared by reactive evaporation
|
journal
|
August 2000 |
|
The partial molar volume and thermal expansivity of TiO 2 in alkali silicate melts: Systematic variation with Ti coordination
|
journal
|
July 2001 |
|
Preparation and properties of binary oxide glasses containing rare earth oxides. [希土類含有高融点酸化物ガラスの作製とその二,三の物性]
|
journal
|
January 1986 |
|
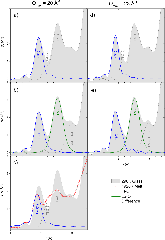
Ti -edge XANES studies of Ti coordination and disorder in oxide compounds: Comparison between theory and experiment
|
journal
|
July 1997 |
|
Surface Tensions and Densities of Melts in TiO<SUB>2</SUB>-BaO and TiO<SUB>2</SUB>-Na<SUB>2</SUB>O Systems [TiO2-BaO系融体およびTiO2-Na2O系融体の表面張力と密度]
|
journal
|
January 1993 |
|
Effect of atomic vibrations on the x-ray absorption spectra at the edge of Al in and of Ti in rutile
|
journal
|
March 2010 |
|
Borate melt structure: a short review
|
journal
|
February 2018 |
|
IFEFFIT : interactive XAFS analysis and FEFF fitting
|
journal
|
March 2001 |
|
An interpretation of glass chemistry in terms of the optical basicity concept
|
journal
|
August 1976 |
|
Magnetosomes could be protective shields against metal stress in magnetotactic bacteria
|
journal
|
July 2020 |