Propane–Water Mixtures Confined within Cylindrical Silica Nanopores: Structural and Dynamical Properties Probed by Molecular Dynamics
- Department of Chemical Engineering, University College London, London WC1E 6BT United Kingdom
- School of Earth Sciences, Ohio State University, Columbus, Ohio 43210 United States
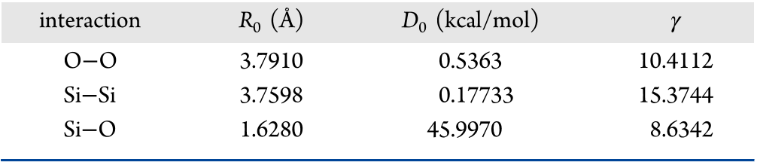
Despite the multiple length and time scales over which fluid-mineral interactions occur, interfacial phenomena control the exchange of matter and impact the nature of multiphase flow, as well as the reactivity of C–O–H fluids in geologic systems. In general, the properties of confined fluids, and their influence on porous geologic phenomena are much less well understood compared to those of bulk fluids. We used equilibrium molecular dynamics simulations to study fluid systems composed of propane and water, at different compositions, confined within cylindrical pores of diameter ~16 Å carved out of amorphous silica. The simulations are conducted within a single cylindrical pore. In the simulated system all the dangling silicon and oxygen atoms were saturated with hydroxyl groups and hydrogen atoms, respectively, yielding a total surface density of 3.8 -OH/nm2. Simulations were performed at 300 K, at different bulk propane pressures, and varying the composition of the system. The structure of the confined fluids was quantified in terms of the molecular distribution of the various molecules within the pore as well as their orientation. This allowed us to quantify the hydrogen bond network and to observe the segregation of propane near the pore center. Transport properties were quantified in terms of the mean square displacement in the direction parallel to the pore axis, which allows us to extract self-diffusion coefficients. The diffusivity of propane in the cylindrical pore was found to depend on pressure, as well as on the amount of water present. It was found that the propane self-diffusion coefficient decreases with increasing water loading because of the formation of water bridges across the silica pores, at sufficiently high water content, which hinder propane transport. The rotational diffusion, the lifespan of hydrogen bonds, and the residence time of water molecules at contact with the silica substrate were quantified from the simulated trajectories using the appropriate autocorrelation functions. The simulations contribute to a better understanding of the molecular phenomena relevant to the behavior of fluids in the subsurface.
- Research Organization:
- The Ohio State Univ., Columbus, OH (United States); Univ. College London (United Kingdom)
- Sponsoring Organization:
- USDOE Office of Science (SC), Basic Energy Sciences (BES); European Union (EU)
- Grant/Contract Number:
- SC0006878; 2013-CIG-631435; 640979
- OSTI ID:
- 1396636
- Alternate ID(s):
- OSTI ID: 1507549
- Journal Information:
- Langmuir, Journal Name: Langmuir Vol. 33 Journal Issue: 42; ISSN 0743-7463
- Publisher:
- American Chemical SocietyCopyright Statement
- Country of Publication:
- United States
- Language:
- English
Web of Science
Effects of water on the stochastic motions of propane confined in MCM-41-S pores
|
journal | January 2019 |
Similar Records
Effects of water on the stochastic motions of propane confined in MCM-41-S pores
Structure and dynamics of ethane confined in silica nanopores in the presence of CO 2