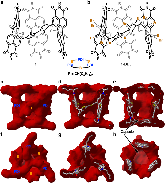
Hollow organic capsules assemble into cellular semiconductors
- Columbia Univ., New York, NY (United States). Dept. of Chemistry
- Columbia Univ., New York, NY (United States). Dept. of Chemistry. Columbia Nano Initiative
- Columbia Univ., New York, NY (United States). Dept. of Applied Physics and Applied Mathematics
- Columbia Univ., New York, NY (United States). Columbia Nano Initiative
- Columbia Univ., New York, NY (United States). Dept. of Applied Physics and Applied Mathematics; Brookhaven National Lab. (BNL), Upton, NY (United States). Condensed Matter Physics and Materials Science Dept.
- Columbia Univ., New York, NY (United States). Dept. of Chemistry; Wuhan Univ. of Science and Technology (China). The State Key Lab. of Refractories and Metallurgy. Inst. of Advanced Materials and Nanotechnology. School of Chemistry and Chemical Engineering
Self-assembly of electroactive molecules is a promising route to new types of functional semiconductors. Here we report a capsule-shaped molecule that assembles itself into a cellular semiconducting material. The interior space of the capsule with a volume of ~415 Å3 is a nanoenvironment that can accommodate a guest. To self-assemble these capsules into electronic materials, we functionalize the thiophene rings with bromines, which encode self-assembly into two-dimensional layers held together through halogen bonding interactions. In the solid state and in films, these two-dimensional layers assemble into the three-dimensional crystalline structure. This hollow material is able to form the active layer in field effect transistor devices. We find that the current of these devices has strong response to the guest’s interaction within the hollow spaces in the film. These devices are remarkable in their ability to distinguish, through their electrical response, between small differences in the guest.
- Research Organization:
- Columbia Univ., New York, NY (United States); Brookhaven National Laboratory (BNL), Upton, NY (United States)
- Sponsoring Organization:
- USDOE Office of Science (SC), Basic Energy Sciences (BES); National Science Foundation (NSF)
- Grant/Contract Number:
- FG02-01ER15264; SC0012704; DMR-1420634
- OSTI ID:
- 1499954
- Journal Information:
- Nature Communications, Vol. 9; ISSN 2041-1723
- Publisher:
- Nature Publishing GroupCopyright Statement
- Country of Publication:
- United States
- Language:
- English
Web of Science
Recyclable mechanoluminescent luminogen: different polymorphs, different self-assembly effects of the thiophene moiety and recovered molecular packing via simple thermal-treatment
|
journal | January 2019 |
The importance of intramolecular conductivity in three dimensional molecular solids
|
journal | January 2019 |
Similar Records
Conjugated Macrocycles in Organic Electronics
Crystalline Nanoporous Frameworks: a Nanolaboratory for Probing Excitonic Device Concepts.