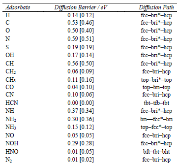
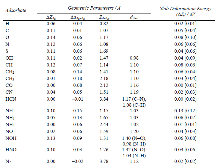
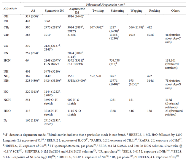
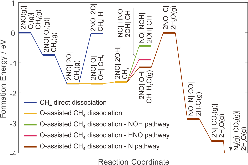
|
What are metal prices like? Co-movement, price cycles and long-run trends
|
journal
|
September 2015 |
|
Why is a Noble Metal Catalytically Active? The Role of the O-Ag Interaction in the Function of Silver as an Oxidation Catalyst
|
journal
|
June 2003 |
|
Silver(I) as a widely applicable, homogeneous catalyst for aerobic oxidation of aldehydes toward carboxylic acids in water—“silver mirror”: From stoichiometric to catalytic
|
journal
|
March 2015 |
|
Colloidal silver catalysts for oxidation of ethylene
|
journal
|
May 1999 |
|
Oxidation of Ethylene over Silver: Adsorption, Kinetics, Catalyst
|
journal
|
January 1980 |
|
Kinetics and mechanism of ethylene oxidation. Reactions of ethylene and ethylene oxide on a silver catalyst
|
journal
|
April 1970 |
|
High temperature partial oxidation reactions over silver catalysts
|
journal
|
November 1999 |
|
The oxidation of methanol on a silver (110) catalyst
|
journal
|
September 1978 |
|
Nanosilver as a new generation of silver catalysts in organic transformations for efficient synthesis of fine chemicals
|
journal
|
January 2015 |
|
Silver-catalysed reactions of alkynes: recent advances
|
journal
|
January 2015 |
|
Silver nanoparticle catalyzed reduction of aromatic nitro compounds
|
journal
|
January 2002 |
|
Design of a Silver-Cerium Dioxide Core-Shell Nanocomposite Catalyst for Chemoselective Reduction Reactions
|
journal
|
October 2011 |
|
Size- and support-dependent silver cluster catalysis for chemoselective hydrogenation of nitroaromatics
|
journal
|
March 2010 |
|
Silver nanoparticles supported on alumina–a highly efficient and selective nanocatalyst for imine reduction
|
journal
|
January 2014 |
|
Electron Microscopy and Catalytic Study of Silver Catalysts: Structure Sensitivity of the Hydrogenation of Crotonaldehyde
|
journal
|
April 1999 |
|
Selective catalytic reduction of NO with methane over Ag-alumina catalysts
|
journal
|
June 2000 |
|
Kinetic modeling of selective catalytic reduction of NOx with octane over Ag–Al2O3
|
journal
|
July 2009 |
|
Hydrogen as a remedy for the detrimental effect of aromatic and cyclic compounds on the HC-SCR over Ag/alumina
|
journal
|
January 2007 |
|
A review of the effect of the addition of hydrogen in the selective catalytic reduction of NO x with hydrocarbons on silver catalysts
|
journal
|
September 2006 |
|
Study of the “Fast SCR”-like mechanism of H2-assisted SCR of NOx with ammonia over Ag/Al2O3
|
journal
|
February 2012 |
|
Alumina-supported silver catalysts for the selective reduction of nitric oxide with propene and oxygen-containing organic compounds
|
journal
|
June 1993 |
|
The Role of Silver for the H2-Effect in H2-Assisted Selective Catalytic Reduction of NO x with NH3 Over Ag/Al2O3
|
journal
|
July 2013 |
|
Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy
|
journal
|
November 2011 |
|
A Plasmonic Photocatalyst Consisting of Silver Nanoparticles Embedded in Titanium Dioxide
|
journal
|
February 2008 |
|
Alloy Formation of Gold−Silver Nanoparticles and the Dependence of the Plasmon Absorption on Their Composition
|
journal
|
May 1999 |
|
Visible-light-enhanced catalytic oxidation reactions on plasmonic silver nanostructures
|
journal
|
May 2011 |
|
Epitaxial growth of a silicene sheet
|
journal
|
November 2010 |
|
Silicene structures on silver surfaces
|
journal
|
July 2012 |
|
Graphene-like silicon nanoribbons on Ag(110): A possible formation of silicene
|
journal
|
May 2010 |
|
Growth and properties of GaN and AlN layers on silver substrates
|
journal
|
November 2005 |
|
Atomic and molecular adsorption on Au(111)
|
journal
|
September 2014 |
|
Atomic and Molecular Adsorption on Ir(111)
|
journal
|
January 2004 |
|
Atomic and molecular adsorption on Pd(111)
|
journal
|
November 2012 |
|
Atomic and molecular adsorption on Pt(111)
|
journal
|
August 2005 |
|
Atomic and Molecular Adsorption on Re(0001)
|
journal
|
October 2013 |
|
Atomic and molecular adsorption on Rh(111)
|
journal
|
October 2002 |
|
Atomic and molecular adsorption on Ru(0001)
|
journal
|
August 2013 |
|
Atomic and molecular adsorption on Fe(110)
|
journal
|
January 2018 |
|
E LECTRONIC S TRUCTURE AND C ATALYSIS ON M ETAL S URFACES
|
journal
|
October 2002 |
|
Special Points in the Brillouin Zone
|
journal
|
December 1973 |
Chemisorption of hydrogen on the Ag(111) surface
- Lee, Geunseop; Sprunger, P. T.; Okada, M.
-
Journal of Vacuum Science & Technology A: Vacuum, Surfaces, and Films, Vol. 12, Issue 4
https://doi.org/10.1116/1.579147
|
journal
|
July 1994 |
|
Atomic and molecular oxygen adsorption on Ag(111)
|
journal
|
July 1985 |
|
The Role of Chlorine in Oxygen Adsorption on Ag(111)
|
journal
|
April 1993 |
|
Oxygen adsorption on Ag(111): A density-functional theory investigation
|
journal
|
January 2002 |
|
The Adsorption of Oxygen on Silver
|
journal
|
October 1964 |
When seeing is not believing: Oxygen on Ag(111), a simple adsorption system?
- Michaelides, Angelos; Reuter, Karsten; Scheffler, Matthias
-
Journal of Vacuum Science & Technology A: Vacuum, Surfaces, and Films, Vol. 23, Issue 6
https://doi.org/10.1116/1.2049302
|
journal
|
November 2005 |
|
Experimental and theoretical study of oxygen adsorption structures on Ag(111)
|
journal
|
August 2009 |
|
Thermally Selective Formation of Subsurface Oxygen in Ag(111) and Consequent Surface Structure
|
journal
|
June 2016 |
|
Structure of : No Silver Oxide
|
journal
|
April 2006 |
|
Ag/Au Mixed Sites Promote Oxidative Coupling of Methanol on the Alloy Surface
|
journal
|
March 2014 |
|
Interaction of hydrogen with the Ag(111) surface
|
journal
|
March 1995 |
|
Reaction of Hydrogen with Ag(111): Binding States, Minimum Energy Paths, and Kinetics
|
journal
|
August 2006 |
|
Endothermic dissociative chemisorption of molecular D2 on Ag(111)
|
journal
|
September 1995 |
|
Atomic deuterium (hydrogen) adsorption on thin silver films
|
journal
|
December 2003 |
|
Interaction of hydrogen with solid surfaces
|
journal
|
July 1988 |
|
Chemisorption geometry of hydrogen on Ni(111): Order and disorder
|
journal
|
May 1979 |
|
Delocalized quantum nature of hydrogen adsorbed on the Rh(111) crystal surface
|
journal
|
November 1986 |
|
The Ag−C (Silver-Carbon) system
|
journal
|
June 1988 |
|
Synthetic Crystals of Silver with Carbon: 3D Epitaxy of Carbon Nanostructures in the Silver Lattice
|
journal
|
July 2015 |
|
Solid-source growth and atomic-scale characterization of graphene on Ag(111)
|
journal
|
November 2013 |
|
Structural and electronic properties of graphene nanoflakes on Au(111) and Ag(111)
|
journal
|
March 2016 |
|
Initial geometries, interaction mechanism and high stability of silicene on Ag(111) surface
|
journal
|
November 2012 |
|
Exploring Ag(111) Substrate for Epitaxially Growing Monolayer Stanene: A First-Principles Study
|
journal
|
July 2016 |
|
Large-Gap Quantum Spin Hall Insulators in Tin Films
|
journal
|
September 2013 |
|
Liquid-source growth of graphene on Ag(001): Liquid-source growth of graphene on Ag(001)
|
journal
|
March 2015 |
|
Sulfur-Induced Reconstruction of Ag(111) Surfaces Studied by DFT
|
journal
|
April 2011 |
|
Novel Self-Organized Structure of a Ag−S Complex on the Ag(111) Surface below Room Temperature
|
journal
|
February 2008 |
|
A single crystal study of the initial stages of silver sulphidation: The chemisorption and reactivity of molecular sulphur (S2) on Ag(111)
|
journal
|
February 1979 |
|
Silver sulphide growth on Ag(111): A medium energy ion scattering study
|
journal
|
August 2010 |
|
Structural Investigation of the Interaction of Molecular Sulfur with Ag(111)
|
journal
|
January 2007 |
|
In Situ STM and Electrochemical Investigation of Sulfur Oxidative Underpotential Deposition on Ag(111)
|
journal
|
June 1997 |
|
Energy dissipation channels in the adsorption of N on Ag(111)
|
journal
|
June 2012 |
|
The interaction of hyperthermal nitrogen with N-covered Ag(111)
|
journal
|
August 2011 |
|
Efficient N 2 Formation on Ag(111) by Eley–Rideal Recombination of Hyperthermal Atoms
|
journal
|
October 2013 |
|
Angular distributions and rovibrational excitation of N2 molecules recombined on N-covered Ag(111) by the Eley–Rideal mechanism
|
journal
|
April 2015 |
|
STM study of hydroxyl formation at
|
journal
|
December 2006 |
|
The adsorption of water and oxygen on Ag(110): A study of the interactions among water molecules, hydroxyl groups, and oxygen atoms
|
journal
|
May 1987 |
|
Adsorption of oxygen on silver single crystal surfaces
|
journal
|
July 1976 |
|
The adsorption of Xe and CO on Ag(111)
|
journal
|
March 1976 |
|
Ammonia adsorption on the Ag(311) surface
|
journal
|
June 1985 |
|
Electron solvation and solvation-induced crystallization of an ammonia film on Ag(111) studied by 2-photon photoemission
|
journal
|
January 2011 |
|
The adsorption and reactions of NO on Ag(111) at 80 K
|
journal
|
November 1989 |
|
Angle-resolved photoemission study of no chemisorption on Ag(111) surface
|
journal
|
October 1988 |
|
Summary Abstract: Decomposition of NO on Ag(111) at low temperatures
|
journal
|
April 1984 |
|
Thermal and Structural Properties of N 2 or CO on Ag(111)
|
journal
|
December 1997 |
|
Hydroxylation of molecularly adsorbed water at Ag(111) and Cu(100) surfaces by dioxygen: photoelectron and vibrational spectroscopic studies
|
journal
|
November 1990 |
|
Generation of Radicals on a Metal Surface from Photoinduced Dissociation of Physisorbed Molecules: CH 2 from H 2 CO on Ag(111)
|
journal
|
January 1996 |
|
Mode-softening of C–H stretch vibration in alkyl groups on Ag(111) and the fluorination effect
|
journal
|
September 2006 |
|
AN INFRARED-SPECTROSCOPIC STUDY OF METHYL IODIDE AND METHYLENE IODIDE ON Ag(111)
|
journal
|
June 2001 |
|
Physisorption of CO on Ag(111): investigation of the monolayer and the multilayer through HREELS, ARUPS, and TDS
|
journal
|
August 1991 |
|
Molecular Constants of Carbon Monoxide at v = 0, 1, 2, and 3: A Vibrational Spectroscopy Experiment in Physical Chemistry
|
journal
|
August 1996 |
|
Anomalously intense Raman scattering at the solid-electrolyte interface
|
journal
|
December 1978 |
|
Electrochemical adsorption of cyanide on Ag(111) in the presence of cetylpyridinium chloride
|
journal
|
October 2004 |
|
Cleavage of N-H bonds by active oxygen on Ag(110): I. Ammonia
|
journal
|
October 1989 |
|
Ammonia adsorption on the Ag(110) surface
|
journal
|
March 1982 |
|
Experimental Vibrational Zero-Point Energies: Diatomic Molecules
|
journal
|
June 2007 |
|
Vibrational and rotational modes of physisorbed H2 and N2 on Ag(111) surfaces
|
journal
|
August 1994 |
|
CO adsorption on close-packed transition and noble metal surfaces: trends from ab initio calculations
|
journal
|
February 2004 |
|
Interaction of CO molecules with surface state electrons on Ag(111)
|
journal
|
October 2005 |
|
STM studies of ordered CO islands on Ag(111)
|
journal
|
April 2005 |
|
Photoemission from Ordered Physisorbed Adsorbate Phases: on Graphite and CO on Ag(111)
|
journal
|
May 1985 |
|
Trends in adsorption of open-shell atoms and small molecular fragments on the Ag(111) surface
|
journal
|
December 2006 |
|
The adsorption of H2O on clean and oxygen-dosed silver single crystal surfaces
|
journal
|
January 1984 |
|
Adsorption, coadsorption, and reactivity of sodium and nitric oxide on Ag(111)
|
journal
|
February 1978 |
|
The chemisorption of spin polarised NO on Ag{111}
|
journal
|
February 1999 |
|
Direct Molecular Imaging of NO Monomers and Dimers and a Surface Reaction on Ag{111}
|
journal
|
March 2001 |
|
Why Is Silver Catalytically Active for NO Reduction? A Unique Pathway via an Inverted (NO) 2 Dimer
|
journal
|
May 2004 |
|
NO Chemisorption and Reactions on Metal Surfaces: A New Perspective
|
journal
|
March 2000 |
|
The vibrational excitation of
|
journal
|
April 1994 |
|
NO2 dissociation on Ag(111) revisited by theory
|
journal
|
March 2008 |
|
Ab initio density-functional study of NO on close-packed transition and noble metal surfaces: I. Molecular adsorption
|
journal
|
December 2005 |
|
Characterization and orientation of adsorbed NO dimers on Ag{111} at low temperatures
|
journal
|
May 1995 |
|
Revisiting NO 2 on Ag( 111 ): a detailed TPD and RAIRS study
|
journal
|
April 2003 |
|
Enhanced Raman effect from cyanide adsorbed on a silver electrode
|
journal
|
February 1980 |
|
Surface-enhanced raman scattering from silver-cyanide and silver-thiocyanate vibrations and the importance of adatoms
|
journal
|
May 1981 |
|
Electrode/electrolyte interphase study using polarization modulated ftir reflection-absorption spectroscopy
|
journal
|
July 1985 |
|
Surface-enhanced Raman spectroscopy of 12CN− and 13CN− adsorbed at silver electrodes
|
journal
|
June 1982 |
|
Thermodynamics of metal cyanide coordination. VI. Copper(I)- and silver(I)-cyanide systems
|
journal
|
January 1967 |
|
Adsorption of complex silver cyanides on Ag(111). Quantum chemical consideration
|
journal
|
January 2016 |
|
CN Stretching Bands in the Raman Spectra of Some Group Ib and Group IIb Complex Cyanides
|
journal
|
September 1960 |
|
Molecular Orbital View of Chemisorbed Carbon Monoxide
|
journal
|
October 1964 |
|
Theory for C—N− and Ag—C vibrational frequency dependence on potential: cyanide on a silver electrode
|
journal
|
August 1981 |
|
The hydrogenation of CN on Pd(111) and Pd(100)
|
journal
|
September 1986 |
|
Molecular Adsorption of HCN on Pt(111) and Cu(100)
|
journal
|
March 1998 |
|
Adsorption and dissociation of HCN on the Pt(111) and Pt(112) surfaces
|
journal
|
September 1988 |
Electron‐induced surface chemistry: Synthesis of NH x fragments on Ag(111)
- Schwaner, A. L.; Pylant, E. D.; White, J. M.
-
Journal of Vacuum Science & Technology A: Vacuum, Surfaces, and Films, Vol. 14, Issue 3
https://doi.org/10.1116/1.579968
|
journal
|
May 1996 |
|
Surface-Mediated NH and N Addition to Styrene on Ag(110)
|
journal
|
November 2006 |
|
Monolayer solid of
|
journal
|
April 1998 |
Population and alignment of N 2 scattered from Ag(111)
- Sitz, Greg O.; Kummel, Andrew C.; Zare, Richard N.
-
Journal of Vacuum Science & Technology A: Vacuum, Surfaces, and Films, Vol. 5, Issue 4
https://doi.org/10.1116/1.574703
|
journal
|
July 1987 |
|
Direct inelastic scattering of N 2 from Ag(111). II. Orientation
|
journal
|
August 1988 |
|
Direct inelastic scattering of N 2 from Ag(111). I. Rotational populations and alignment
|
journal
|
August 1988 |
|
Elucidation of Pathways for NO Electroreduction on Pt(111) from First Principles
|
journal
|
June 2015 |
|
Density Functional Theory Calculations and Analysis of Reaction Pathways for Reduction of Nitric Oxide by Hydrogen on Pt(111)
|
journal
|
September 2014 |
|
NO dissociation and reduction by H 2 on Pd(1 1 1): A first-principles study
|
journal
|
February 2015 |
|
Review of Ag/Al2O3-Reductant System in the Selective Catalytic Reduction of NO x
|
journal
|
January 2008 |
|
On the performance of Ag/Al 2 O 3 as a HC-SCR catalyst – influence of silver loading, morphology and nature of the reductant
|
journal
|
January 2013 |
|
New Insights into the Active Surface Species of Silver Alumina Catalysts in the Selective Catalytic Reduction of NO
|
journal
|
May 2010 |
|
Alumina-supported Catalysts for the Selective Reduction of Nitric Oxide by Propene
|
journal
|
September 1993 |
|
Unique catalytic features of Ag nanoclusters for selective NOx reduction and green chemical reactions
|
journal
|
January 2011 |
|
Microkinetic modeling for hydrocarbon (HC)-based selective catalytic reduction (SCR) of NOx on a silver-based catalyst
|
journal
|
July 2009 |
|
Mechanistic Aspects of the Selective Reduction of NO by Propene over Alumina and Silver–Alumina Catalysts
|
journal
|
October 1999 |
|
Catalytic solutions to reduce pollutants
|
journal
|
October 1997 |
|
Kinetic considerations of H2 assisted hydrocarbon selective catalytic reduction of NO over Ag/Al2O3
|
journal
|
May 2006 |
|
Promotion effect of H2 on the low temperature activity of the selective reduction of NO by light hydrocarbons over Ag/Al2O3
|
journal
|
May 2003 |
|
Novel Pd–Au/TiO2 catalyst for the selective catalytic reduction of NOx by H2
|
journal
|
December 2014 |
|
Hydrogen Dissociation and Spillover on Individual Isolated Palladium Atoms
|
journal
|
December 2009 |
|
An Atomic-Scale View of Palladium Alloys and their Ability to Dissociate Molecular Hydrogen
|
journal
|
October 2010 |
|
Hydrogen adsorption on and spillover from Au- and Cu-supported Pt3 and Pd3 clusters: a density functional study
|
journal
|
January 2012 |
|
A benchmark database for adsorption bond energies to transition metal surfaces and comparison to selected DFT functionals
|
journal
|
October 2015 |