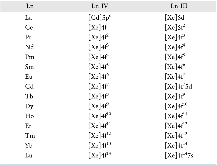
Establishing Cost-Effective Computational Models for the Prediction of Lanthanoid Binding in [Ln(NO 3 )] 2+ (with Ln = La to Lu)
- Research Information Technology Services, University of North Texas, 225 S. Avenue B, Denton, Texas 76201, United States, Institute for Nuclear Security, University of Tennessee, 1640 Cumberland Avenue, Knoxville, Tennessee 37996, United States
- Institute for Nuclear Security, University of Tennessee, 1640 Cumberland Avenue, Knoxville, Tennessee 37996, United States
- Department of Nuclear Engineering, University of Tennessee, 301 Middle Dr., Pasqua Nuclear Engineering Bldg., Knoxville, Tennessee 37996, United States
- Institute for Nuclear Security, University of Tennessee, 1640 Cumberland Avenue, Knoxville, Tennessee 37996, United States, Department of Nuclear Engineering, University of Tennessee, 301 Middle Dr., Pasqua Nuclear Engineering Bldg., Knoxville, Tennessee 37996, United States, Radiochemistry Center of Excellence (RCOE), University of Tennessee, 1508 Middle Dr., Ferris Hall, Knoxville, Tennessee 37996, United States, Y-12 National Security Complex, Oak Ridge, Tennessee 37830, United States
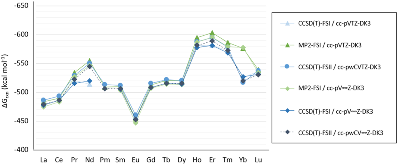
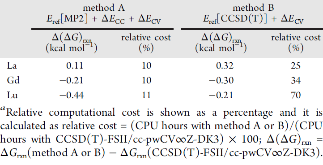
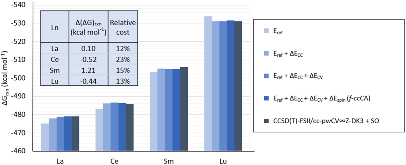
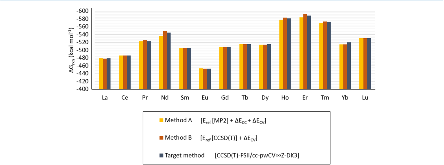
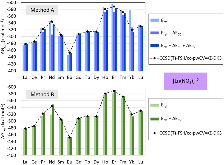
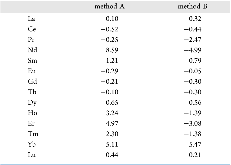
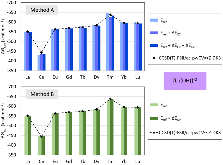
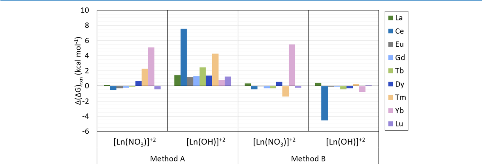
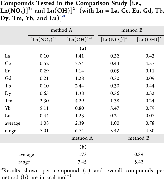
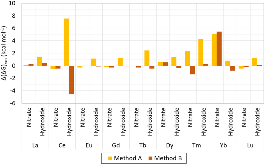
Evaluating the efficiency of predictive methods is critical to the processes of upscaling laboratory processes to full-scale operations on an industrial scale. With regard to separation of lanthanoids, there is a considerable motivation to optimize these processes because of immediate use in nuclear fuel cycle operations, nuclear forensics applications, and rare-earth metal recovery. Efficient predictive capabilities in Gibbs free energies of reaction are essential to optimize separations and ligand design for selective binding needed for various radiochemical applications such as nuclear fuel disposition and recycling of lanthanoid fission products into useful radioisotope products. Ligand design is essential for selective binding of lanthanoids, as separating contiguous lanthanoids is challenging because of the similar behavior these elements exhibit. Modeling including electronic structure calculations of lanthanoid-containing compounds is particularly challenging because of the associated computational cost encountered with the number of electrons correlated in these systems and relativistic considerations. This study evaluates the predictive capabilities of various ab initio methods in the calculation of Gibbs free energies of reaction for [Ln(NO3)]2+ compounds (with Ln = La to Lu), as nitrates are critical in traditional separation processes utilizing nitric acid. The composite methodologies evaluated predict Gibbs free energies of reaction for [Ln(NO3)]2+ compounds within 5 kcal mol–1 in most cases from the target method [CCSD(T)-FSII/cc-pwCV∞Z-DK3+SO] at a fraction of the computational cost.
- Research Organization:
- Univ. of Tennessee, Knoxville, TN (United States)
- Sponsoring Organization:
- USDOE National Nuclear Security Administration (NNSA); USDOE Office of Science (SC); National Science Foundation (NSF)
- Grant/Contract Number:
- NA0001983; AC02-05CH11231; ACI-1548562
- OSTI ID:
- 1491051
- Alternate ID(s):
- OSTI ID: 1508790
- Journal Information:
- ACS Omega, Journal Name: ACS Omega Vol. 4 Journal Issue: 1; ISSN 2470-1343
- Publisher:
- American Chemical Society (ACS)Copyright Statement
- Country of Publication:
- United States
- Language:
- English
Web of Science
Similar Records
Synthesis, properties, and x-ray structures of the lanthanide {eta}{sup 6}-arene-bridged aryloxide dimers Ln{sub 2}(O-2,6-i-Pr{sub 2}H{sub 3}){sub 6} and their Lewis base adducts Ln(O-2,6-i-Pr{sub 2}C{sub 6}H{sub 3}){sub 3}(THF){sub 2}(Ln = Pr, Nd, Sm, Gd, Er, Yb, Lu)
Synthesis and characterization of Ln(B{sub 0.5}Mn{sub 0.5})O{sub 3} (Ln-lanthanoid; B = Ni, Co) perovskites