Effect of alumina incorporation on the sulfur tolerance of the dual-catalyst aftertreatment system for reduction of nitrogen oxides under lean conditions
- The Ohio State Univ., Columbus, OH (United States). William G. Lowrie Dept. of Chemical and Biomolecular Engineering; Oak Ridge National Lab. (ORNL), Oak Ridge, TN (United States)
- The Ohio State Univ., Columbus, OH (United States). William G. Lowrie Dept. of Chemical and Biomolecular Engineering
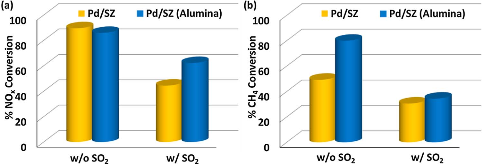
Presence of sulfur or sulfur additives in natural gas lead to the formation of sulfur dioxide in the exhaust stream of natural gas-fired lean-burn engines. The sulfur tolerance and hydrothermal stability of the dual-catalyst aftertreatment system comprising a reduction catalyst (palladium supported on sulfated zirconia- Pd/SZ) and an oxidation catalyst (cobalt oxide supported on ceria- CoOx/CeO2) for NOx reduction under lean-burn conditions has been tested under simulated engine exhaust conditions. Alumina-incorporated Pd/SZ (Pd/SZ(Alumina)) reduction catalyst as part of the dual catalyst bed demonstrated superior sulfur tolerance in the presence of 5 ppm SO2 in the feed. The ratio of the reduction to oxidation catalyst was also seen to affect the hydrothermal stability under wet conditions with SO2 in the reaction medium. The effect of hydrocarbon and water concentration in the reaction mixture on the sulfur tolerance of the dual-catalyst bed was studied. Increasing the reaction temperature from 450°C to 500°C demonstrated very stable NOx conversion and improved CH4 conversions when the dual-catalyst bed was kept on-stream for 40 h-1. X-ray photoelectron spectroscopy on samples poisoned with SO2 ex-situ revealed the deposition of surface sulfates on the Pd/SZ catalyst samples and a combination of sulfates and sulfites on the CoOx/CeO2 on treatment with SO2. The presence of alumina in the Pd/SZ sample protects the support from SO2 poisoning as the oxidation state of the zirconia support remained largely unchanged on poisoning while in the CoOx/CeO2 sample, sulfur poisoning likely affects the cerium redox cycle (Ce3+-Ce4+). 27Al magic angle spinning nuclear magnetic resonance (MAS NMR) revealed redistribution of the octahedral and tetrahedral Al-O coordination on SO2 poisoning. Due to association with deposited sulfates on the catalyst, shielding effect on the Al nuclei resulted in an increased octahedral Al-O coordination in the poisoned sample.
- Research Organization:
- Oak Ridge National Lab. (ORNL), Oak Ridge, TN (United States); The Ohio State Univ., Columbus, OH (United States)
- Sponsoring Organization:
- USDOE; Caterpillar Inc., Peoria, IL (United States)
- Grant/Contract Number:
- AC05-00OR22725
- OSTI ID:
- 1471840
- Journal Information:
- Catalysis Today, Vol. 320; ISSN 0920-5861
- Publisher:
- ElsevierCopyright Statement
- Country of Publication:
- United States
- Language:
- English
Web of Science
Similar Records
Investigation of Aging Mechanisms in Lean NOx Traps
Characteristics of Desulfation Behavior for Presulfated Pt-BaO/CeO2 Lean NOx Trap Catalyst: The Role of the CeO2 Support