Experimental and Theoretical Insights into the Potential of V2O3 Surface Coatings for Hydrogen Permeable Vanadium Membranes
Abstract
A grand challenge of vanadium-based H2 permeable membranes is the development of effective, cheap, and stable catalysts to facilitate H2 dissociation and recombination. This article investigates a facile air treatment to form catalytically active vanadium oxide on the surfaces of dense vanadium foils. The treatment consisted of short air exposure followed by H2 reduction at 823 K, which produced a well-faceted and nanocrystalline V2O3 layer on the foil surfaces. The resulting membranes display stable H2 permeability of 2 ± 0.25 × 10-8 mol·m-1·s-1·Pa-0.5, but transient declines in permeation are observed when operated at both elevated and reduced temperatures. DFT calculations revealed that V2O3 (0001) surfaces display barriers and adsorption energies for H2 dissociation/recombination that are comparable to known H2 activation catalysts. It was found that H2 dissociation is expected to proceed spontaneously on metal-terminated V2O3, with recombinative-desorption anticipated as the rate limiting step.
- Authors:
-
- Colorado School of Mines, Golden, CO (United States). Dept. of Chemical and Biological Engineering
- Publication Date:
- Research Org.:
- Colorado School of Mines, Golden, CO (United States)
- Sponsoring Org.:
- USDOE Advanced Research Projects Agency - Energy (ARPA-E); USDOE Office of Nuclear Energy (NE); National Science Foundation (NSF)
- OSTI Identifier:
- 1457549
- Grant/Contract Number:
- AR0000785; 1512172; 0000785
- Resource Type:
- Journal Article: Accepted Manuscript
- Journal Name:
- Journal of Physical Chemistry. C
- Additional Journal Information:
- Journal Volume: 122; Journal Issue: 6; Journal ID: ISSN 1932-7447
- Publisher:
- American Chemical Society
- Country of Publication:
- United States
- Language:
- English
- Subject:
- 08 HYDROGEN; 36 MATERIALS SCIENCE; Vanadium oxide catalyst; Hydrogen purification; Group V metals; Metallic membranes; DFT simulation
Citation Formats
Fuerst, Thomas F., Petsalis, Elias P., Lundin, Sean-Thomas B., Wilcox, Jennifer, Way, J. Douglas, and Wolden, Colin A. Experimental and Theoretical Insights into the Potential of V2O3 Surface Coatings for Hydrogen Permeable Vanadium Membranes. United States: N. p., 2018.
Web. doi:10.1021/acs.jpcc.7b12132.
Fuerst, Thomas F., Petsalis, Elias P., Lundin, Sean-Thomas B., Wilcox, Jennifer, Way, J. Douglas, & Wolden, Colin A. Experimental and Theoretical Insights into the Potential of V2O3 Surface Coatings for Hydrogen Permeable Vanadium Membranes. United States. https://doi.org/10.1021/acs.jpcc.7b12132
Fuerst, Thomas F., Petsalis, Elias P., Lundin, Sean-Thomas B., Wilcox, Jennifer, Way, J. Douglas, and Wolden, Colin A. 2018.
"Experimental and Theoretical Insights into the Potential of V2O3 Surface Coatings for Hydrogen Permeable Vanadium Membranes". United States. https://doi.org/10.1021/acs.jpcc.7b12132. https://www.osti.gov/servlets/purl/1457549.
@article{osti_1457549,
title = {Experimental and Theoretical Insights into the Potential of V2O3 Surface Coatings for Hydrogen Permeable Vanadium Membranes},
author = {Fuerst, Thomas F. and Petsalis, Elias P. and Lundin, Sean-Thomas B. and Wilcox, Jennifer and Way, J. Douglas and Wolden, Colin A.},
abstractNote = {A grand challenge of vanadium-based H2 permeable membranes is the development of effective, cheap, and stable catalysts to facilitate H2 dissociation and recombination. This article investigates a facile air treatment to form catalytically active vanadium oxide on the surfaces of dense vanadium foils. The treatment consisted of short air exposure followed by H2 reduction at 823 K, which produced a well-faceted and nanocrystalline V2O3 layer on the foil surfaces. The resulting membranes display stable H2 permeability of 2 ± 0.25 × 10-8 mol·m-1·s-1·Pa-0.5, but transient declines in permeation are observed when operated at both elevated and reduced temperatures. DFT calculations revealed that V2O3 (0001) surfaces display barriers and adsorption energies for H2 dissociation/recombination that are comparable to known H2 activation catalysts. It was found that H2 dissociation is expected to proceed spontaneously on metal-terminated V2O3, with recombinative-desorption anticipated as the rate limiting step.},
doi = {10.1021/acs.jpcc.7b12132},
url = {https://www.osti.gov/biblio/1457549},
journal = {Journal of Physical Chemistry. C},
issn = {1932-7447},
number = 6,
volume = 122,
place = {United States},
year = {Tue Jan 23 00:00:00 EST 2018},
month = {Tue Jan 23 00:00:00 EST 2018}
}
Web of Science
Figures / Tables:
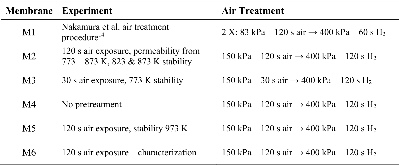 Table 1: List of membranes fabricated and tested in this study.
Table 1: List of membranes fabricated and tested in this study.