A Comparative Study of Hydrodeoxygenation of Furfural Over Fe/Pt(111) and Fe/Mo2C Surfaces
- Columbia Univ., New York, NY (United States)
- Jiangsu Univ., Zhenjiang (People's Republic of China)
- Columbia Univ., New York, NY (United States); Brookhaven National Lab. (BNL), Upton, NY (United States)
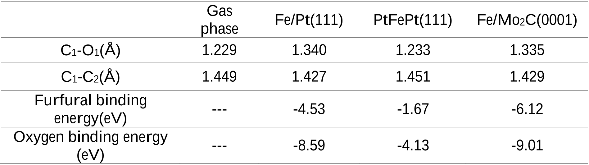
It is desirable to convert biomass-derived furfural to 2-methylfuran through the hydrodeoxygenation (HDO) reaction using an inexpensive catalyst with high stability. In this work, Mo2C was used as an alternative substrate to replace precious Pt to support monolayer Fe for the HDO reaction of furfural. The HDO activity and stability of Fe/Pt(111) and Fe/Mo2C/Mo(110) surfaces were compared. Density functional theory (DFT) calculations and vibrational spectroscopy results indicated that both surfaces bonded to furfural with similar adsorption geometries and should be active toward the furfural HDO reaction. Temperature Programmed Desorption (TPD) experiments confirmed a similar HDO activity between the two surfaces, with Fe/Mo2C/Mo(110) being more thermally stable than Fe/Pt(111). The combined theoretical and experimental results demonstrated that Fe/Mo2C should be a promising non-precious metal catalyst for the HDO reaction of furfural to produce 2-methylfuran.
- Research Organization:
- Energy Frontier Research Centers (EFRC) (United States). Catalysis Center for Energy Innovation (CCEI); Brookhaven National Lab. (BNL), Upton, NY (United States); Columbia Univ., New York, NY (United States)
- Sponsoring Organization:
- USDOE Office of Science (SC), Basic Energy Sciences (BES)
- Grant/Contract Number:
- SC0012704; AC02-05CH11231; SC0001004
- OSTI ID:
- 1454816
- Alternate ID(s):
- OSTI ID: 1659740
- Report Number(s):
- BNL-205764-2018-JAAM
- Journal Information:
- Topics in Catalysis, Vol. 61, Issue 5-6; ISSN 1022-5528
- Publisher:
- SpringerCopyright Statement
- Country of Publication:
- United States
- Language:
- English
Web of Science
Vibrational Spectroscopic Characterization of Glycerol Reaction Pathways over Metal‐Modified Molybdenum Carbide Surfaces
|
journal | October 2019 |
Similar Records
Cobalt-modified molybdenum carbide as a selective catalyst for hydrodeoxygenation of furfural
Reaction pathways of furfural, furfuryl alcohol and 2-methylfuran on Cu(111) and NiCu bimetallic surfaces