Influence of Hydrogen Sulfide Exposure on the Transport and Structural Properties of the Metal–Organic Framework ZIF-8
- Univ. of Florida, Gainesville, FL (United States). Dept. of Chemical Engineering
- Univ. of Wisconsin, Madison, WI (United States). Theoretical Chemistry Inst. Dept. of Chemistry
- Georgia Inst. of Technology, Atlanta, GA (United States). School of Chemical and Biomolecular Engineering
- Univ. of Florida, Gainesville, FL (United States). National High Magnetic Field Lab.
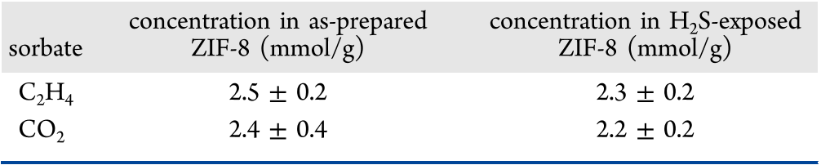
In this paper, the interaction between hydrogen sulfide and ZIF-8 was studied via structural characterizations and guest molecule diffusion measurements. It was found that hydrogen sulfide reacts with the ZIF-8 external particle surface to form a surface barrier that excludes the uptake of larger molecules (ethanol) and slows down the uptake of smaller molecules (carbon dioxide). Nonetheless, bulk transport properties were unaltered, as supported by pulsed field gradient nuclear magnetic resonance studies. Dispersion-corrected density functional theory calculations revealed that H2S is consumed by reactions occurring at the ZIF external surface. These reactions result in water and defect formation, both of which were found to be exothermic and independent of both crystallographic facets ({001} and {110}) and surface termination. Finally, we concluded that these surface reactions lead to structural and chemical changes to the ZIF-8 external surface that generate surface barriers to molecular transport.
- Research Organization:
- Georgia Institute of Technology, Atlanta, GA (United States); Univ. of Florida, Gainesville, FL (United States); Energy Frontier Research Centers (EFRC) (United States). Center for Understanding and Control of Acid Gas-induced Evolution of Materials for Energy (UNCAGE-ME)
- Sponsoring Organization:
- USDOE Office of Science (SC), Basic Energy Sciences (BES); National Science Foundation (NSF)
- Grant/Contract Number:
- SC0012577; ECCS-1542174; DMR-1157490
- OSTI ID:
- 1428044
- Journal Information:
- Journal of Physical Chemistry. C, Vol. 122, Issue 13; ISSN 1932-7447
- Publisher:
- American Chemical SocietyCopyright Statement
- Country of Publication:
- United States
- Language:
- English
Web of Science
Similar Records
Anomaly in the Chain Length Dependence of n-Alkane Diffusion in ZIF-4 Metal-Organic Frameworks
Metal-organic and zeolite imidazolate frameworks (MOFs and ZIFs) for highly selective separations