Structural Evidence of a Major Conformational Change Triggered by Substrate Binding in DapE Enzymes: Impact on the Catalytic Mechanism
- Argonne National Lab. (ANL), Argonne, IL (United States). Midwest Center for Structural Genomics. Structural Biology Center. Biosciences Division
- Loyola Univ. Chicago, IL (United States). Dept. of Chemistry and Biochemistry
- Marquette Univ., Milwaukee, WI (United States). Dept. of Chemistry
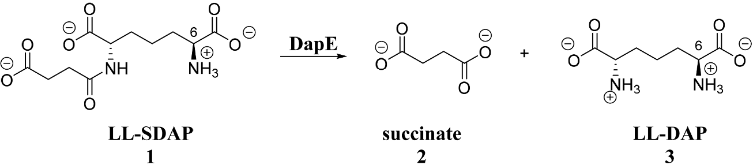
In this paper, the X-ray crystal structure of the dapE-encoded N-succinyl-l,l-diaminopimelic acid desuccinylase from Haemophilus influenzae (HiDapE) bound by the products of hydrolysis, succinic acid and l,l-DAP, was determined at 1.95 Å. Surprisingly, the structure bound to the products revealed that HiDapE undergoes a significant conformational change in which the catalytic domain rotates ~50° and shifts ~10.1 Å (as measured at the position of the Zn atoms) relative to the dimerization domain. This heretofore unobserved closed conformation revealed significant movements within the catalytic domain compared to that of wild-type HiDapE, which results in effectively closing off access to the dinuclear Zn(II) active site with the succinate carboxylate moiety bridging the dinculear Zn(II) cluster in a μ-1,3 fashion forming a bis(μ-carboxylato)dizinc(II) core with a Zn–Zn distance of 3.8 Å. Surprisingly, His194.B, which is located on the dimerization domain of the opposing chain ~10.1 Å from the dinuclear Zn(II) active site, forms a hydrogen bond (2.9 Å) with the oxygen atom of succinic acid bound to Zn2, forming an oxyanion hole. As the closed structure forms upon substrate binding, the movement of His194.B by more than ~10 Å is critical, based on site-directed mutagenesis data, for activation of the scissile carbonyl carbon of the substrate for nucleophilic attack by a hydroxide nucleophile. Employing the HiDapE product-bound structure as the starting point, a reverse engineering approach called product-based transition-state modeling provided structural models for each major catalytic step. Finally, these data provide insight into the catalytic reaction mechanism and also the future design of new, potent inhibitors of DapE enzymes.
- Research Organization:
- Argonne National Laboratory (ANL), Argonne, IL (United States); Loyola Univ. Chicago, IL (United States); Marquette Univ., Milwaukee, WI (United States)
- Sponsoring Organization:
- USDOE Office of Science (SC), Biological and Environmental Research (BER); National Inst. of Health (NIH) (United States); National Science Foundation (NSF); Todd Wehr Foundation (United States)
- Grant/Contract Number:
- AC02-06CH11357; HHSN272200700058C; HHSN272201200026C; CHE-1412443
- OSTI ID:
- 1427504
- Journal Information:
- Biochemistry, Vol. 57, Issue 5; ISSN 0006-2960
- Publisher:
- American Chemical Society (ACS)Copyright Statement
- Country of Publication:
- United States
- Language:
- English
Web of Science
Practical spectrophotometric assay for the dapE-encoded N-succinyl-L,L-diaminopimelic acid desuccinylase, a potential antibiotic target
|
journal | April 2018 |
Similar Records
Indoline-6-Sulfonamide Inhibitors of the Bacterial Enzyme DapE
Mono-N-protected amino acid ligands stabilize dimeric palladium(II) complexes of importance to C–H functionalization