Nucleation of stoichiometric compounds from liquid: Role of the kinetic factor
- Ames Lab., Ames, IA (United States). Division of Materials Sciences and Engineering
- Ames Lab., Ames, IA (United States). Division of Materials Sciences and Engineering; Iowa State Univ., Ames, IA (United States). Dept. of Physics; Univ. of Science and Technology of China, Hefei (China). Hefei National Lab. for Physical Sciences at the Microscale, and Dept. of Physics
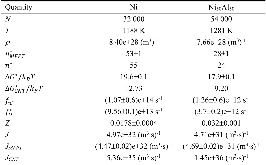
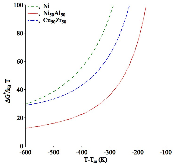
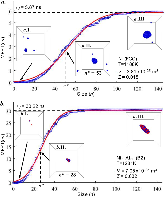
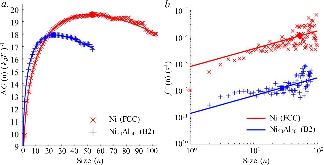
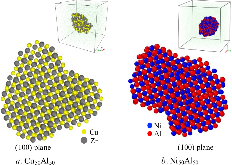
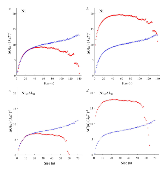
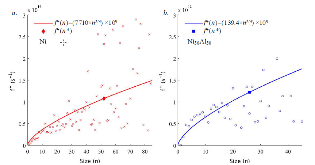
The nucleation rate depends on the free-energy barrier and the kinetic factor. While the role of the free energy barrier is a text-book subject, the importance of the kinetic factor is frequently underestimated. Here in this study, we applied the mean first-passage time method, to obtain the free-energy landscape and kinetic factor directly from the molecular dynamics (MD) simulations of the nucleation of the face-centered cubic (fcc) phase in the pure Ni and the B2 phases in the Ni50Al50 and Cu50Zr50 alloys. The obtained data show that while the free-energy barrier for nucleation is higher in pure Ni the nucleation rate is considerably lower in the Ni50Al50 alloy. This result can be explained by the slow attachment kinetics in the N i 50 A l 50 alloy, which was related to the ordered nature of the B2 phase. Even smaller fraction of the antisite defects in the C u 50 Z r 50 alloy leads to such a slow attachment kinetics that the nucleation is never observed for this alloy in the course of the MD simulation. Finally, this is consistent with the experimental facts that the Cu50Zr50 alloy is a good glass forming alloy and the Ni50Al50 alloy is not. Thus the present study demonstrates that the atom attachment rate can be the critical factor that controls the nucleation process under certain conditions.
- Research Organization:
- Ames Laboratory (AMES), Ames, IA (United States)
- Sponsoring Organization:
- USDOE Office of Science (SC), Basic Energy Sciences (BES)
- Grant/Contract Number:
- AC02-07CH11358
- OSTI ID:
- 1425479
- Alternate ID(s):
- OSTI ID: 1421880
- Report Number(s):
- IS-J-9586; PRMHAR; TRN: US1802111
- Journal Information:
- Physical Review Materials, Vol. 2, Issue 2; ISSN 2475-9953
- Publisher:
- American Physical Society (APS)Copyright Statement
- Country of Publication:
- United States
- Language:
- English
Web of Science
Similar Records
Annual Report: Fuels (30 September 2012)
Competitive B2 and B33 Nucleation during Solidification of Ni 50 Zr 50 Alloy: Molecular Dynamics Simulation and Classical Nucleation Theory