Oxygen-induced defects at the lead halide perovskite/graphene oxide interfaces
Abstract
Here, graphene oxide or its reduced derivative (GO/RGO) replace metal oxides in perovskite photovoltaics to achieve energy band alignment for minimization of the energy barriers at the film interfaces allowing efficient charge transport, and eliminate stability issues. However, the power conversion efficiencies fall in a wide range (~0.6–18%). Therefore, the perovskite growth and nucleation on GO/RGO require fundamental understanding to improve device function for controlled fabrication, which remain a major challenge. We analyze the surface morphology and crystallization of the lead halide perovskites (MAPbX3) at 20–300 °C on GO using X-ray diffraction and photoelectron spectroscopy. To determine defect mechanisms and their composition, we perform in situ transmission infrared and micro Raman spectroscopy, and the cross-sectional scanning microscopy that captures interfacial imperfections with the oxygen defects. We demonstrate the oxygen-induced defects at the MAPbX3/GO interfaces that initiate at room temperature, and occur through the nucleophilic substitution reactions. Unexpectedly, structural defects nucleate in GO forming chemically reduced GO, and modify the surface morphology that yield a poor perovskite growth. Our theoretical studies also reveal that energetically favorable, exothermic reactions between the halides of the perovskite precursors and the oxygen groups of GO generate acidic reaction by-products (i.e. HX), that confirm the formationmore »
- Authors:
-
- Argonne National Lab. (ANL), Lemont, IL (United States)
- Ulsan National Institute of Science and Technology, Ulsan (Korea)
- Publication Date:
- Research Org.:
- Argonne National Lab. (ANL), Argonne, IL (United States)
- Sponsoring Org.:
- USDOE Office of Science (SC), Basic Energy Sciences (BES)
- OSTI Identifier:
- 1422395
- Grant/Contract Number:
- AC02-06CH11357
- Resource Type:
- Journal Article: Accepted Manuscript
- Journal Name:
- Journal of Materials Chemistry. A
- Additional Journal Information:
- Journal Volume: 6; Journal Issue: 4; Journal ID: ISSN 2050-7488
- Publisher:
- Royal Society of Chemistry
- Country of Publication:
- United States
- Language:
- English
- Subject:
- 37 INORGANIC, ORGANIC, PHYSICAL, AND ANALYTICAL CHEMISTRY
Citation Formats
Acik, Muge, Park, In Kee, Koritala, Rachel E., Lee, Geunsik, and Rosenberg, Richard A. Oxygen-induced defects at the lead halide perovskite/graphene oxide interfaces. United States: N. p., 2017.
Web. doi:10.1039/c7ta10010h.
Acik, Muge, Park, In Kee, Koritala, Rachel E., Lee, Geunsik, & Rosenberg, Richard A. Oxygen-induced defects at the lead halide perovskite/graphene oxide interfaces. United States. https://doi.org/10.1039/c7ta10010h
Acik, Muge, Park, In Kee, Koritala, Rachel E., Lee, Geunsik, and Rosenberg, Richard A. 2017.
"Oxygen-induced defects at the lead halide perovskite/graphene oxide interfaces". United States. https://doi.org/10.1039/c7ta10010h. https://www.osti.gov/servlets/purl/1422395.
@article{osti_1422395,
title = {Oxygen-induced defects at the lead halide perovskite/graphene oxide interfaces},
author = {Acik, Muge and Park, In Kee and Koritala, Rachel E. and Lee, Geunsik and Rosenberg, Richard A.},
abstractNote = {Here, graphene oxide or its reduced derivative (GO/RGO) replace metal oxides in perovskite photovoltaics to achieve energy band alignment for minimization of the energy barriers at the film interfaces allowing efficient charge transport, and eliminate stability issues. However, the power conversion efficiencies fall in a wide range (~0.6–18%). Therefore, the perovskite growth and nucleation on GO/RGO require fundamental understanding to improve device function for controlled fabrication, which remain a major challenge. We analyze the surface morphology and crystallization of the lead halide perovskites (MAPbX3) at 20–300 °C on GO using X-ray diffraction and photoelectron spectroscopy. To determine defect mechanisms and their composition, we perform in situ transmission infrared and micro Raman spectroscopy, and the cross-sectional scanning microscopy that captures interfacial imperfections with the oxygen defects. We demonstrate the oxygen-induced defects at the MAPbX3/GO interfaces that initiate at room temperature, and occur through the nucleophilic substitution reactions. Unexpectedly, structural defects nucleate in GO forming chemically reduced GO, and modify the surface morphology that yield a poor perovskite growth. Our theoretical studies also reveal that energetically favorable, exothermic reactions between the halides of the perovskite precursors and the oxygen groups of GO generate acidic reaction by-products (i.e. HX), that confirm the formation of oxygen-induced defects.},
doi = {10.1039/c7ta10010h},
url = {https://www.osti.gov/biblio/1422395},
journal = {Journal of Materials Chemistry. A},
issn = {2050-7488},
number = 4,
volume = 6,
place = {United States},
year = {Thu Dec 21 00:00:00 EST 2017},
month = {Thu Dec 21 00:00:00 EST 2017}
}
Web of Science
Figures / Tables:
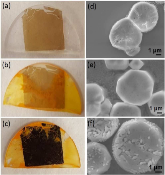 Figure 1: Photos of a) as-prepared GO (3-5 layers) deposited on a quartz piece, b) after spin coating (40 μl solution, at a spin rate of 4000 rpm, 30s) of methylammonium bromide and lead bromide at 40 wt.% in DMF at room temperature, and c) after annealing at 130°C formore »
Figure 1: Photos of a) as-prepared GO (3-5 layers) deposited on a quartz piece, b) after spin coating (40 μl solution, at a spin rate of 4000 rpm, 30s) of methylammonium bromide and lead bromide at 40 wt.% in DMF at room temperature, and c) after annealing at 130°C formore »
Works referenced in this record:
Intriguing Optoelectronic Properties of Metal Halide Perovskites
journal, June 2016
- Manser, Joseph S.; Christians, Jeffrey A.; Kamat, Prashant V.
- Chemical Reviews, Vol. 116, Issue 21
Dipolar Aprotic Solvents in Bimolecular Aromatic Nucleophilic Substitution Reactions 1
journal, January 1961
- Miller, J.; Parker, Alan J.
- Journal of the American Chemical Society, Vol. 83, Issue 1
Ferroelectric Graphene–Perovskite Interfaces
journal, June 2015
- Volonakis, George; Giustino, Feliciano
- The Journal of Physical Chemistry Letters, Vol. 6, Issue 13
H2TPP Organocatalysis in Mild and Highly Regioselective Ring Opening of Epoxides to Halo Alcohols by Means of Halogen Elements
journal, May 2012
- Torabi, Parviz; Azizian, Javad; Zomorodbakhsh, Shahab
- Molecules, Vol. 17, Issue 5
Investigation on thermal evaporated CH 3 NH 3 PbI 3 thin films
journal, September 2015
- Li, Youzhen; Xu, Xuemei; Wang, Chenggong
- AIP Advances, Vol. 5, Issue 9
Opening of epoxides in the presence of iodine
journal, March 1971
- Jewell, J. S.; Szarek, W. A.
- Carbohydrate Research, Vol. 16, Issue 1
Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells
journal, May 2009
- Kojima, Akihiro; Teshima, Kenjiro; Shirai, Yasuo
- Journal of the American Chemical Society, Vol. 131, Issue 17, p. 6050-6051
Stabilization of Organic-Inorganic Perovskite Layers by Partial Substitution of Iodide by Bromide in Methylammonium Lead Iodide
journal, March 2016
- Ruess, Raffael; Benfer, Felix; Böcher, Felix
- ChemPhysChem, Vol. 17, Issue 10
Interface engineering of highly efficient perovskite solar cells
journal, July 2014
- Zhou, H.; Chen, Q.; Li, G.
- Science, Vol. 345, Issue 6196
Perovskite solar cells with CuSCN hole extraction layers yield stabilized efficiencies greater than 20%
journal, September 2017
- Arora, Neha; Dar, M. Ibrahim; Hinderhofer, Alexander
- Science, Vol. 358, Issue 6364
Graphene: Status and Prospects
journal, June 2009
- Geim, A. K.
- Science, Vol. 324, Issue 5934, p. 1530-1534
Efficient planar heterojunction perovskite solar cells employing graphene oxide as hole conductor
journal, January 2014
- Wu, Zhongwei; Bai, Sai; Xiang, Jian
- Nanoscale, Vol. 6, Issue 18
Reproducible Fabrication of Efficient Perovskite-based Solar Cells: X-ray Crystallographic Studies on the Formation of CH 3 NH 3 PbI 3 Layers
journal, May 2014
- Wakamiya, Atsushi; Endo, Masaru; Sasamori, Takahiro
- Chemistry Letters, Vol. 43, Issue 5
Graphene in perovskite solar cells: device design, characterization and implementation
journal, January 2016
- Acik, Muge; Darling, Seth B.
- Journal of Materials Chemistry A, Vol. 4, Issue 17
π-Bonding-dominated energy gaps in graphene oxide
journal, January 2016
- Thuy Tran, Ngoc Thanh; Lin, Shih-Yang; Glukhova, Olga E.
- RSC Advances, Vol. 6, Issue 29
Reduced Graphene Oxide/Mesoporous TiO 2 Nanocomposite Based Perovskite Solar Cells
journal, October 2015
- Han, Gill Sang; Song, Young Hyun; Jin, Young Un
- ACS Applied Materials & Interfaces, Vol. 7, Issue 42
Interfacial Oxygen Vacancies as a Potential Cause of Hysteresis in Perovskite Solar Cells
journal, January 2016
- Zhang, Fan; Ma, Wei; Guo, Haizhong
- Chemistry of Materials, Vol. 28, Issue 3
Role of Chloride in the Morphological Evolution of Organo-Lead Halide Perovskite Thin Films
journal, October 2014
- Williams, Spencer T.; Zuo, Fan; Chueh, Chu-Chen
- ACS Nano, Vol. 8, Issue 10
Encapsulation for long-term stability enhancement of perovskite solar cells
journal, December 2016
- Matteocci, Fabio; Cinà, Lucio; Lamanna, Enrico
- Nano Energy, Vol. 30
Solvent engineering for high-performance inorganic–organic hybrid perovskite solar cells
journal, July 2014
- Jeon, Nam Joong; Noh, Jun Hong; Kim, Young Chan
- Nature Materials, Vol. 13, Issue 9, p. 897-903
Structure and Growth Control of Organic-Inorganic Halide Perovskites for Optoelectronics: From Polycrystalline Films to Single Crystals
journal, March 2016
- Chen, Yani; He, Minhong; Peng, Jiajun
- Advanced Science, Vol. 3, Issue 4
The Importance of Moisture in Hybrid Lead Halide Perovskite Thin Film Fabrication
journal, August 2015
- Eperon, Giles E.; Habisreutinger, Severin N.; Leijtens, Tomas
- ACS Nano, Vol. 9, Issue 9
Ionic liquid-assisted growth of methylammonium lead iodide spherical nanoparticles by a simple spin-coating method and photovoltaic properties of perovskite solar cells
journal, January 2015
- Shahiduzzaman, M.; Yamamoto, Kohei; Furumoto, Yoshikazu
- RSC Advances, Vol. 5, Issue 95
Infrared spectroscopy of liquid water–N,N-dimethylformamide mixtures
journal, February 2009
- Biliškov, Nikola; Baranović, Goran
- Journal of Molecular Liquids, Vol. 144, Issue 3
Infrared Spectra and Characteristic Frequencies of Inorganic Ions
journal, August 1952
- Miller, F. A.; Wilkins, C. H.
- Analytical Chemistry, Vol. 24, Issue 8
The Role of Intercalated Water in Multilayered Graphene Oxide
journal, October 2010
- Acik, Muge; Mattevi, Cecilia; Gong, Cheng
- ACS Nano, Vol. 4, Issue 10
The Role of Oxygen during Thermal Reduction of Graphene Oxide Studied by Infrared Absorption Spectroscopy
journal, September 2011
- Acik, Muge; Lee, Geunsik; Mattevi, Cecilia
- The Journal of Physical Chemistry C, Vol. 115, Issue 40
Efficiency and Stability Enhancement in Perovskite Solar Cells by Inserting Lithium-Neutralized Graphene Oxide as Electron Transporting Layer
journal, February 2016
- Agresti, Antonio; Pescetelli, Sara; Cinà, Lucio
- Advanced Functional Materials, Vol. 26, Issue 16
Crystal growth engineering for high efficiency perovskite solar cells
journal, January 2016
- Park, Nam-Gyu
- CrystEngComm, Vol. 18, Issue 32
Selective dissolution of halide perovskites as a step towards recycling solar cells
journal, May 2016
- Kim, Byeong Jo; Kim, Dong Hoe; Kwon, Seung Lee
- Nature Communications, Vol. 7, Issue 1
Parameters that control and influence the organo-metal halide perovskite crystallization and morphology
journal, March 2016
- Cohen, Bat-El; Etgar, Lioz
- Frontiers of Optoelectronics, Vol. 9, Issue 1
Phase transition kinetics and surface binding states of methylammonium lead iodide perovskite
journal, January 2016
- Rajendra Kumar, G.; Dennyson Savariraj, A.; Karthick, S. N.
- Physical Chemistry Chemical Physics, Vol. 18, Issue 10
Interface Engineering of Perovskite Solar Cell Using a Reduced-Graphene Scaffold
journal, August 2016
- Tavakoli, Mohammad Mahdi; Tavakoli, Rouhollah; Hasanzadeh, Soheil
- The Journal of Physical Chemistry C, Vol. 120, Issue 35
Graphene-Based Electron Transport Layers in Perovskite Solar Cells: A Step-Up for an Efficient Carrier Collection
journal, September 2017
- Biccari, Francesco; Gabelloni, Fabio; Burzi, Erica
- Advanced Energy Materials, Vol. 7, Issue 22
Material and Device Stability in Perovskite Solar Cells
journal, August 2016
- Kim, Hui-Seon; Seo, Ja-Young; Park, Nam-Gyu
- ChemSusChem, Vol. 9, Issue 18
Transformation of the Excited State and Photovoltaic Efficiency of CH 3 NH 3 PbI 3 Perovskite upon Controlled Exposure to Humidified Air
journal, January 2015
- Christians, Jeffrey A.; Miranda Herrera, Pierre A.; Kamat, Prashant V.
- Journal of the American Chemical Society, Vol. 137, Issue 4
Progress, challenges and perspectives in flexible perovskite solar cells
journal, January 2016
- Di Giacomo, Francesco; Fakharuddin, Azhar; Jose, Rajan
- Energy & Environmental Science, Vol. 9, Issue 10
Investigation on the nucleation and growth mechanisms of perovskite formation in the two-step solution process
conference, June 2016
- Watthage, Suneth C.; Song, Zhaoning; Liyanage, Geethika K.
- 2016 IEEE 43rd Photovoltaic Specialists Conference (PVSC)
Is Excess PbI 2 Beneficial for Perovskite Solar Cell Performance?
journal, January 2016
- Liu, Fangzhou; Dong, Qi; Wong, Man Kwong
- Advanced Energy Materials, Vol. 6, Issue 7
Stable and null current hysteresis perovskite solar cells based nitrogen doped graphene oxide nanoribbons hole transport layer
journal, June 2016
- Kim, Jeongmo; Mat Teridi, Mohd Asri; Mohd Yusoff, Abd. Rashid bin
- Scientific Reports, Vol. 6, Issue 1
Beneficial Role of Reduced Graphene Oxide for Electron Extraction in Highly Efficient Perovskite Solar Cells
journal, September 2016
- Cho, Kyung Taek; Grancini, Giulia; Lee, Yonghui
- ChemSusChem, Vol. 9, Issue 21
Efficiency Enhancement of Perovskite Solar Cells through Fast Electron Extraction: The Role of Graphene Quantum Dots
journal, February 2014
- Zhu, Zonglong; Ma, Jiani; Wang, Zilong
- Journal of the American Chemical Society, Vol. 136, Issue 10
The effect of skin-depth interfacial defect layer in perovskite solar cell
journal, July 2016
- Gebremichael, Bizuneh; Mola, Genene Tessema
- Applied Physics B, Vol. 122, Issue 8
Thermally Stable Mesoporous Perovskite Solar Cells Incorporating Low-Temperature Processed Graphene/Polymer Electron Transporting Layer
journal, October 2016
- Tong, Shi Wun; Balapanuru, Janardhan; Fu, Deyi
- ACS Applied Materials & Interfaces, Vol. 8, Issue 43
Reduction of Lead Oxide (PbO 2 ) by Iodide and Formation of Iodoform in the PbO 2 /I − /NOM System
journal, April 2008
- Lin, Yi-Pin; Washburn, Michael P.; Valentine, Richard L.
- Environmental Science & Technology, Vol. 42, Issue 8
Evolution of Chemical Composition, Morphology, and Photovoltaic Efficiency of CH 3 NH 3 PbI 3 Perovskite under Ambient Conditions
journal, December 2015
- Huang, Weixin; Manser, Joseph S.; Kamat, Prashant V.
- Chemistry of Materials, Vol. 28, Issue 1
Defects in perovskite-halides and their effects in solar cells
journal, October 2016
- Ball, James M.; Petrozza, Annamaria
- Nature Energy, Vol. 1, Issue 11
Efficient perovskite solar cells fabricated using an aqueous lead nitrate precursor
journal, January 2015
- Hsieh, Tsung-Yu; Wei, Tzu-Chien; Wu, Kuan-Lin
- Chemical Communications, Vol. 51, Issue 68
Preferential Facet Growth of Methylammonium Lead Halide Single Crystals Promoted by Halide Coordination
journal, August 2016
- Zhang, Yi; Huang, Fuqiang; Mi, Qixi
- Chemistry Letters, Vol. 45, Issue 8
Graphene-Perovskite Solar Cells Exceed 18 % Efficiency: A Stability Study
journal, September 2016
- Agresti, Antonio; Pescetelli, Sara; Taheri, Babak
- ChemSusChem, Vol. 9, Issue 18
Studies of the reactions HBr(HI)+e to or from Br-(I-)+H using the FALP and SIFT techniques
journal, September 1987
- Smith, D.; Adams, N. G.
- Journal of Physics B: Atomic and Molecular Physics, Vol. 20, Issue 18
On the Thermal and Thermodynamic (In)Stability of Methylammonium Lead Halide Perovskites
journal, August 2016
- Brunetti, Bruno; Cavallo, Carmen; Ciccioli, Andrea
- Scientific Reports, Vol. 6, Issue 1
Infrared Spectroscopic Study of Vibrational Modes in Methylammonium Lead Halide Perovskites
journal, July 2015
- Glaser, Tobias; Müller, Christian; Sendner, Michael
- The Journal of Physical Chemistry Letters, Vol. 6, Issue 15
Infrared Spectroscopy of Hydrated Bicarbonate Anion Clusters: HCO 3 − (H 2 O) 1−10
journal, January 2010
- Garand, Etienne; Wende, Torsten; Goebbert, Daniel J.
- Journal of the American Chemical Society, Vol. 132, Issue 2
Graphene oxide/PEDOT:PSS composite hole transport layer for efficient and stable planar heterojunction perovskite solar cells
journal, January 2016
- Lee, Da-Young; Na, Seok-In; Kim, Seok-Soon
- Nanoscale, Vol. 8, Issue 3
Intrinsic Thermal Instability of Methylammonium Lead Trihalide Perovskite
journal, June 2015
- Conings, Bert; Drijkoningen, Jeroen; Gauquelin, Nicolas
- Advanced Energy Materials, Vol. 5, Issue 15
Lewis Acid–Base Adduct Approach for High Efficiency Perovskite Solar Cells
journal, January 2016
- Lee, Jin-Wook; Kim, Hui-Seon; Park, Nam-Gyu
- Accounts of Chemical Research, Vol. 49, Issue 2
Thermal decomposition of basic lead carbonate
journal, March 1976
- Sarig, S.; Kahana, F.
- Thermochimica Acta, Vol. 14, Issue 3
The benefits of graphene for hybrid perovskite solar cells
journal, December 2016
- Bouclé, Johann; Herlin-Boime, Nathalie
- Synthetic Metals, Vol. 222
Making and Breaking of Lead Halide Perovskites
journal, January 2016
- Manser, Joseph S.; Saidaminov, Makhsud I.; Christians, Jeffrey A.
- Accounts of Chemical Research, Vol. 49, Issue 2
Chiral Selective Chemistry Induced by Natural Selection of Spin-Polarized Electrons
journal, May 2015
- Rosenberg, Richard A.; Mishra, Debabrata; Naaman, Ron
- Angewandte Chemie International Edition, Vol. 54, Issue 25
Solution processed graphene structures for perovskite solar cells
journal, January 2016
- Batmunkh, Munkhbayar; Shearer, Cameron J.; Biggs, Mark J.
- Journal of Materials Chemistry A, Vol. 4, Issue 7
Enhancing Efficiency of Perovskite Solar Cells via N-doped Graphene: Crystal Modification and Surface Passivation
journal, August 2016
- Hadadian, Mahboubeh; Correa-Baena, Juan-Pablo; Goharshadi, Elaheh K.
- Advanced Materials, Vol. 28, Issue 39
Large fill-factor bilayer iodine perovskite solar cells fabricated by a low-temperature solution-process
journal, January 2014
- Wang, Qi; Shao, Yuchuan; Dong, Qingfeng
- Energy Environ. Sci., Vol. 7, Issue 7
Enhanced planar perovskite solar cells with efficiency exceeding 16% via reducing the oxygen vacancy defect state in titanium oxide electrode
journal, January 2017
- Du, Yangyang; Cai, Hongkun; Wu, Yunhao
- Physical Chemistry Chemical Physics, Vol. 19, Issue 21
Role of interface in stability of perovskite solar cells
journal, February 2017
- Manspeaker, Chris; Venkatesan, Swaminathan; Zakhidov, Alex
- Current Opinion in Chemical Engineering, Vol. 15
Efficient and Highly Air Stable Planar Inverted Perovskite Solar Cells with Reduced Graphene Oxide Doped PCBM Electron Transporting Layer
journal, December 2016
- Kakavelakis, George; Maksudov, Temur; Konios, Dimitrios
- Advanced Energy Materials, Vol. 7, Issue 7
Graphene Interface Engineering for Perovskite Solar Modules: 12.6% Power Conversion Efficiency over 50 cm 2 Active Area
journal, December 2016
- Agresti, Antonio; Pescetelli, Sara; Palma, Alessandro L.
- ACS Energy Letters, Vol. 2, Issue 1
Large-area ultrathin films of reduced graphene oxide as a transparent and flexible electronic material
journal, April 2008
- Eda, Goki; Fanchini, Giovanni; Chhowalla, Manish
- Nature Nanotechnology, Vol. 3, Issue 5, p. 270-274
Thermal Assisted Oxygen Annealing for High Efficiency Planar CH3NH3PbI3 Perovskite Solar Cells
journal, October 2014
- Ren, Zhiwei; Ng, Annie; Shen, Qian
- Scientific Reports, Vol. 4, Issue 1
Highly efficient and stable planar perovskite solar cells with reduced graphene oxide nanosheets as electrode interlayer
journal, March 2015
- Yeo, Jun-Seok; Kang, Rira; Lee, Sehyun
- Nano Energy, Vol. 12
Observation and Mediation of the Presence of Metallic Lead in Organic–Inorganic Perovskite Films
journal, June 2015
- Sadoughi, Golnaz; Starr, David E.; Handick, Evelyn
- ACS Applied Materials & Interfaces, Vol. 7, Issue 24
An XPS study of the adsorption of lead on goethite (α-FeOOH)
journal, October 1998
- Abdel-Samad, Hesham; Watson, Philip R.
- Applied Surface Science, Vol. 136, Issue 1-2
Graphene oxide as dual functional interface modifier for improving wettability and retarding recombination in hybrid perovskite solar cells
journal, January 2014
- Li, Wenzhe; Dong, Haopeng; Guo, Xudong
- J. Mater. Chem. A, Vol. 2, Issue 47
High-Performance Perovskite Solar Cells Engineered by an Ammonia Modified Graphene Oxide Interfacial Layer
journal, June 2016
- Feng, Shanglei; Yang, Yingguo; Li, Meng
- ACS Applied Materials & Interfaces, Vol. 8, Issue 23
The solubility of some sparingly soluble lead salts: An evaluation of the solubility in water and aqueous electrolyte solution
journal, July 1980
- Clever, H. Lawrence; Johnston, Francis J.
- Journal of Physical and Chemical Reference Data, Vol. 9, Issue 3
Structural evolution during the reduction of chemically derived graphene oxide
journal, June 2010
- Bagri, Akbar; Mattevi, Cecilia; Acik, Muge
- Nature Chemistry, Vol. 2, Issue 7, p. 581-587
Works referencing / citing this record:
Morphology and Interface Engineering for Organic Metal Halide Perovskite-Based Photovoltaic Cells
journal, May 2018
- Wei, Qi; Bi, Huan; Yan, Su
- Advanced Materials Interfaces, Vol. 5, Issue 14
Doping and Photon Induced Defect Healing of Hybrid Perovskite Thin Films: An Approach Towards Efficient Light Emitting Diodes
journal, March 2019
- Kanwat, Anil; Choi, Won‐Chul; Seth, Sudipta
- ChemNanoMat, Vol. 5, Issue 5
Enhancement in lifespan of halide perovskite solar cells
journal, January 2019
- Wang, Qiong; Phung, Nga; Di Girolamo, Diego
- Energy & Environmental Science, Vol. 12, Issue 3
Highly photoluminescent, dense solid films from organic-capped CH 3 NH 3 PbBr 3 perovskite colloids
journal, January 2018
- González-Carrero, Soranyel; Martínez-Sarti, Laura; Sessolo, Michele
- Journal of Materials Chemistry C, Vol. 6, Issue 25
Ultrathin lead bromide perovskite platelets spotted with europium( ii ) bromide dots
journal, January 2019
- Rosa-Pardo, Ignacio; Pocoví-Martínez, Salvador; Arenal, Raul
- Nanoscale, Vol. 11, Issue 39
Formation of a photoactive quasi-2D formamidinium lead iodide perovskite in water
journal, January 2019
- Jana, Atanu; Ba, Qiankai; Nissimagoudar, Arun S.
- Journal of Materials Chemistry A, Vol. 7, Issue 45