Formation of NO+ and its possible roles during the selective catalytic reduction of NOx with NH3 on Cu-CHA catalysts
- Johnson Matthey Inc., Wayne, PA (United States)
- Pacific Northwest National Lab. (PNNL), Richland, WA (United States). Inst. for Integrated Catalysis
- Johnson Matthey Inc., Wayne, PA (United States); Pacific Northwest National Lab. (PNNL), Richland, WA (United States). Inst. for Integrated Catalysis
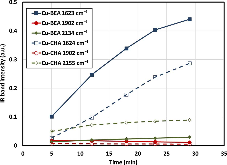
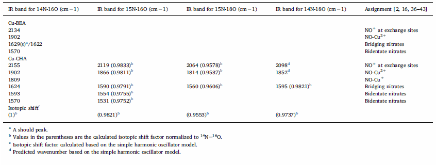
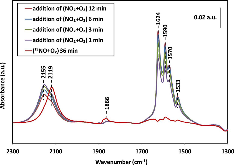
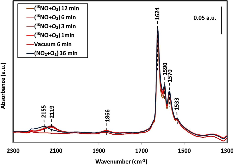
The catalytic activities of small-pore Cu-CHA and large-pore Cu-BEA catalysts for the selective catalytic reduction of NO with NH3 were measured at a very high flow rate. Cu-CHA clearly exhibited much higher intrinsic SCR activity and lower N2O selectivity. In situ DRIFT spectra were recorded during the adsorption and desorption following NO and (NO+O2) exposure to fully oxidized samples in a flow cell. The results are in agreement with what we have reported previously based on in situ transmission IR studies of partially reduced samples. Both suggest that different SCR reaction pathways might exist on these two catalysts and that NO+ could be an important reaction intermediate for Cu-CHA. Detailed IR studies with various isotopically labeled gas mixtures of (NO+O2), (15NO+O2), (NO+18O2) and (15N18O+O2) were conducted to understand the origin of the surface adsorption complexes on Cu-CHA. Formation of NO+ was not the consequence of a simple charge transfer reaction, NO+Cu2+=NO+ + Cu+. Instead, O2 was found to be essential in changing the oxidation state of N from +2 to +3 although it did not participate in new N$$-$$O bond formation. In conclusion, the majority of the adsorbed NO+ maintained its isotopic origin of the feed gas.
- Research Organization:
- Pacific Northwest National Lab. (PNNL), Richland, WA (United States)
- Sponsoring Organization:
- USDOE Office of Science (SC), Biological and Environmental Research (BER); USDOE Office of Energy Efficiency and Renewable Energy (EERE), Vehicle Technologies Office (EE-3V)
- Grant/Contract Number:
- AC05-76RL01830
- OSTI ID:
- 1415772
- Alternate ID(s):
- OSTI ID: 1702798
- Report Number(s):
- PNNL-SA-129558; PII: S0920586117308441; TRN: US1800847
- Journal Information:
- Catalysis Today, Vol. 320; ISSN 0920-5861
- Publisher:
- ElsevierCopyright Statement
- Country of Publication:
- United States
- Language:
- English
Web of Science
Similar Records
Mechanistic studies of NH3-assisted reduction of mononuclear Cu(ii) cation sites in Cu-CHA zeolites
Investigation of the reaction pathways in selective catalytic reduction of NO with NH{sub 3} over V{sub 2}O{sub 5} catalysts: Isotopic labeling studies using {sup 18}O{sub 2}, {sup 15}NH{sub 3}, {sup 15}NO, and {sup 15}N{sup 18}O{sup 1}