Reduction of CO2 to methanol using aluminum ester FLPs
- Los Alamos National Lab. (LANL), Los Alamos, NM (United States)
- The Univ. of Alabama, Tuscaloosa, AL (United States)
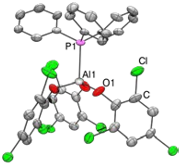
Herein we report the synthesis of Al-based esters containing halogenated benzene rings. These Lewis acids were paired with phosphines to form frustrated Lewis pairs (FLPs) which could subsequently bind CO2. While these FLPs were not sufficiently water-stable to catalyze the reduction of CO2 to MeOH using NH3BH3 as the reductant, we examine the effect of varying Lewis acid strength. Frustrated Lewis pairs (FLPs) are combinations of Lewis acids and Lewis bases where the acid and base are either sterically or geometrically restricted from interacting as strongly as their electronic structures would allow. This effect leads to enhanced reactivity towards small molecules and, consequently, interest in their potential as metal-free catalysts [1], [2], [3], [4] and [5]. Furthermore, to-date, the biggest success has been based around the ability of a myriad of systems to heterolytically cleave H2 and perform catalytic hydrogenations [2] and [3].
- Research Organization:
- Los Alamos National Lab. (LANL), Los Alamos, NM (United States)
- Sponsoring Organization:
- USDOE Laboratory Directed Research and Development (LDRD) Program
- Grant/Contract Number:
- AC52-06NA25396; LDRD 20120197ER
- OSTI ID:
- 1329898
- Alternate ID(s):
- OSTI ID: 1780435
- Report Number(s):
- LA-UR-15-26338
- Journal Information:
- Inorganic Chemistry Communications, Vol. 61, Issue C; ISSN 1387-7003
- Publisher:
- ElsevierCopyright Statement
- Country of Publication:
- United States
- Language:
- English
Web of Science
Diphosphination of CO 2 and CS 2 mediated by frustrated Lewis pairs – catalytic route to phosphanyl derivatives of formic and dithioformic acid
|
journal | January 2019 |
CO 2 Capture by 2-(Methylamino)pyridine Ligated Aluminum Alkyl Complexes: CO 2 Capture by 2-(Methylamino)pyridine Ligated Aluminum Alkyl Complexes
|
journal | May 2020 |
Similar Records
High-Pressure Hydrogen Interactions with Polyaminoborane and Polyiminoborane
Small Molecule Activation with Intramolecular “Inverse” Frustrated Lewis Pairs