Synthesis, crystal growth, structural and magnetic characterization of NH4MCl2(HCOO), M=(Fe, Co, Ni)
Abstract
In this paper, an ambient-pressure solution route and an improved solvothermal synthetic method have been developed to produce polycrystalline powders and large single crystals of NH4MCl2(HCOO) (M=Fe, Co, Ni). The magnetic structure of the 1D linear chain compound NH4FeCl2(HCOO) has been determined by low-temperature neutron powder diffraction, revealing ferromagnetic intra-chain interactions and antiferromagnetic inter-chain interactions. Finally, the newly-reported Co and Ni analogs are isostructural with NH4FeCl2(HCOO), but there are significant differences in the magnetic properties of each compound; the Ni analog behaves similarly to the Fe compound but with stronger magnetic coupling, exhibiting antiferromagnetic ordering (TN=8.5 K) and a broad metamagnetic transition between 2 and 5 T, while the Co analog does not order magnetically above 2 K, despite strong antiferromagnetic nearest-neighbor interactions.
- Authors:
-
- Univ. of California, Davis, CA (United States). Dept. of Chemistry
- Oak Ridge National Lab. (ORNL), Oak Ridge, TN (United States). Quantum Condensed Matter Division
- Univ. of Tennessee Space Inst. (UTSI), Tullahoma, TN (United States). Mechanical, Aerospace and Biomedical Engineering Dept.
- Publication Date:
- Research Org.:
- Oak Ridge National Lab. (ORNL), Oak Ridge, TN (United States)
- Sponsoring Org.:
- USDOE Office of Science (SC), Basic Energy Sciences (BES); Univ. of California, Davis (United States)
- OSTI Identifier:
- 1261278
- Alternate Identifier(s):
- OSTI ID: 1244122
- Grant/Contract Number:
- AC05-00OR22725
- Resource Type:
- Journal Article: Accepted Manuscript
- Journal Name:
- Journal of Solid State Chemistry
- Additional Journal Information:
- Journal Volume: 236; Journal ID: ISSN 0022-4596
- Publisher:
- Elsevier
- Country of Publication:
- United States
- Language:
- English
- Subject:
- 37 INORGANIC, ORGANIC, PHYSICAL, AND ANALYTICAL CHEMISTRY; 75 CONDENSED MATTER PHYSICS, SUPERCONDUCTIVITY AND SUPERFLUIDITY; Solvothermal synthesis; Crystal growth; Low-dimensional magnetism; Neutron diffraction; Mossbauer spectroscopy; Magnetic structure
Citation Formats
Greenfield, Joshua T., Ovidiu Garlea, V., Kamali, Saeed, Chen, Michael, and Kovnir, Kirill. Synthesis, crystal growth, structural and magnetic characterization of NH4MCl2(HCOO), M=(Fe, Co, Ni). United States: N. p., 2015.
Web. doi:10.1016/j.jssc.2015.09.016.
Greenfield, Joshua T., Ovidiu Garlea, V., Kamali, Saeed, Chen, Michael, & Kovnir, Kirill. Synthesis, crystal growth, structural and magnetic characterization of NH4MCl2(HCOO), M=(Fe, Co, Ni). United States. https://doi.org/10.1016/j.jssc.2015.09.016
Greenfield, Joshua T., Ovidiu Garlea, V., Kamali, Saeed, Chen, Michael, and Kovnir, Kirill. 2015.
"Synthesis, crystal growth, structural and magnetic characterization of NH4MCl2(HCOO), M=(Fe, Co, Ni)". United States. https://doi.org/10.1016/j.jssc.2015.09.016. https://www.osti.gov/servlets/purl/1261278.
@article{osti_1261278,
title = {Synthesis, crystal growth, structural and magnetic characterization of NH4MCl2(HCOO), M=(Fe, Co, Ni)},
author = {Greenfield, Joshua T. and Ovidiu Garlea, V. and Kamali, Saeed and Chen, Michael and Kovnir, Kirill},
abstractNote = {In this paper, an ambient-pressure solution route and an improved solvothermal synthetic method have been developed to produce polycrystalline powders and large single crystals of NH4MCl2(HCOO) (M=Fe, Co, Ni). The magnetic structure of the 1D linear chain compound NH4FeCl2(HCOO) has been determined by low-temperature neutron powder diffraction, revealing ferromagnetic intra-chain interactions and antiferromagnetic inter-chain interactions. Finally, the newly-reported Co and Ni analogs are isostructural with NH4FeCl2(HCOO), but there are significant differences in the magnetic properties of each compound; the Ni analog behaves similarly to the Fe compound but with stronger magnetic coupling, exhibiting antiferromagnetic ordering (TN=8.5 K) and a broad metamagnetic transition between 2 and 5 T, while the Co analog does not order magnetically above 2 K, despite strong antiferromagnetic nearest-neighbor interactions.},
doi = {10.1016/j.jssc.2015.09.016},
url = {https://www.osti.gov/biblio/1261278},
journal = {Journal of Solid State Chemistry},
issn = {0022-4596},
number = ,
volume = 236,
place = {United States},
year = {Thu Sep 24 00:00:00 EDT 2015},
month = {Thu Sep 24 00:00:00 EDT 2015}
}
Web of Science
Figures / Tables:
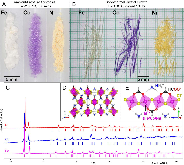 Figure 1.: A: Polycrystalline powders and B: single crystals of NH4MCl2(HCOO) (M = Fe, Co, Ni), produced from ambient-pressure synthesis and solvothermal recrystallization, respectively. C: Room-temperature PXRD patterns for each compound, with calculated peak positions (>1% total intensity) indicated by tick marks below the patterns. Fe: magenta; Co: blue; Ni:more »
Figure 1.: A: Polycrystalline powders and B: single crystals of NH4MCl2(HCOO) (M = Fe, Co, Ni), produced from ambient-pressure synthesis and solvothermal recrystallization, respectively. C: Room-temperature PXRD patterns for each compound, with calculated peak positions (>1% total intensity) indicated by tick marks below the patterns. Fe: magenta; Co: blue; Ni:more »
Works referenced in this record:
The Role of Hydrothermal Synthesis in Preparative Chemistry
journal, December 1985
- Rabenau, Albrecht
- Angewandte Chemie International Edition in English, Vol. 24, Issue 12
Iron( II ) Formate [Fe(O 2 CH) 2 ]·1/3HCO 2 H: A Mesoporous Magnet − Solvothermal Syntheses and Crystal Structures of the Isomorphous Framework Metal( II ) Formates [M(O 2 CH) 2 ]· n (Solvent) (M = Fe, Co, Ni, Zn, Mg): Iron( II ) Formate [Fe(O 2 CH) 2 ]·1/3HCO 2 H: A Mesoporous Magnet
journal, February 2005
- Viertelhaus, Martin; Adler, Peter; Clérac, Rodolphe
- European Journal of Inorganic Chemistry, Vol. 2005, Issue 4
Formate-Based Magnetic Metal-Organic Frameworks Templated by Protonated Amines
journal, March 2010
- Wang, Zheming; Hu, Keli; Gao, Song
- Advanced Materials, Vol. 22, Issue 13
The magneto-structural correlation of two novel 1D antiferromagnetic chains with different magnetic behaviors
journal, November 2011
- Li, Bo; Zhang, Xiaoying; Tian, Jumei
- Polyhedron, Vol. 30, Issue 18
Constructing magnetic molecular solids by employing three-atom ligands as bridges
journal, January 2008
- Wang, Xin-Yi; Wang, Zhe-Ming; Gao, Song
- Chem. Commun., Issue 3
A Highly Anisotropic Cobalt(II)-Based Single-Chain Magnet: Exploration of Spin Canting in an Antiferromagnetic Array
journal, October 2008
- Palii, Andrei V.; Reu, Oleg S.; Ostrovsky, Sergei M.
- Journal of the American Chemical Society, Vol. 130, Issue 44
Coexistence of Magnetic and Electric Orderings in the Metal–Formate Frameworks of [NH 4 ][M(HCOO) 3 ]
journal, September 2011
- Xu, Guan-Cheng; Zhang, Wen; Ma, Xiao-Ming
- Journal of the American Chemical Society, Vol. 133, Issue 38
NH 4 FeCl 2 (HCOO): Synthesis, Structure, and Magnetism of a Novel Low-Dimensional Magnetic Material
journal, February 2014
- Greenfield, Joshua T.; Kamali, Saeed; Izquierdo, Nezhueyotl
- Inorganic Chemistry, Vol. 53, Issue 6
A short history of SHELX
journal, December 2007
- Sheldrick, George M.
- Acta Crystallographica Section A Foundations of Crystallography, Vol. 64, Issue 1, p. 112-122
Recent advances in magnetic structure determination by neutron powder diffraction
journal, October 1993
- Rodríguez-Carvajal, Juan
- Physica B: Condensed Matter, Vol. 192, Issue 1-2
A new protocol for the determination of magnetic structures using simulated annealing and representational analysis (SARAh)
journal, March 2000
- Wills, A. S.
- Physica B: Condensed Matter, Vol. 276-278
Symmetry-Based Computational Tools for Magnetic Crystallography
journal, July 2015
- Perez-Mato, J. M.; Gallego, S. V.; Tasci, E. S.
- Annual Review of Materials Research, Vol. 45, Issue 1
Analytical expression for the Mössbauer line shape of 57Fe in the presence of mixed hyperfine interactions
journal, May 1985
- Blaes, Nikolaus; Fischer, Harald; Gonser, Ulrich
- Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms, Vol. 9, Issue 2
Two Chain Compounds of [M(N 3 ) 2 (HCOO)][(CH 3 ) 2 NH 2 ] (M = Fe and Co) with a Mixed Azido/Formato Bridge Displaying Metamagnetic Behavior
journal, April 2006
- Liu, Tao; Zhang, Yanjuan; Wang, Zheming
- Inorganic Chemistry, Vol. 45, Issue 7
Synthesis of [NH 4 ]MnCl 2 (OAc) and [NH 4 ] 2 MnCl 4 (H 2 O) 2 by Solvothermal Dehydration and Structure/Property Correlations in a One-Dimensional Antiferromagnet
journal, April 2004
- Martin, James D.; Hess, Ryan F.; Boyle, Paul D.
- Inorganic Chemistry, Vol. 43, Issue 10
Cobalt(II) metamagnet built from ferromagnetic chains with mixed bis(azido)(carboxylate) bridges
journal, June 2012
- Wen, Yan-Qing; Ma, Yu; Wang, Yan-Qin
- Inorganic Chemistry Communications, Vol. 20
Works referencing / citing this record:
Three coordination frameworks with copper formate based low dimensional motifs: synthesis, structure and magnetic properties
journal, January 2017
- Bovill, Sally M.; Dixey, Richard J. C.; Saines, Paul J.
- CrystEngComm, Vol. 19, Issue 13
Neutron Instruments for Research in Coordination Chemistry: Neutron Instruments for Research in Coordination Chemistry
journal, January 2019
- Xue, Zi-Ling; Ramirez-Cuesta, Anibal J.; Brown, Craig M.
- European Journal of Inorganic Chemistry, Vol. 2019, Issue 8
Insertion of CS 2 into the Mg–H bond: synthesis and structural characterization of the magnesium dithioformate complex, [Tism PriBenz ]Mg(κ 2 -S 2 CH)
journal, January 2018
- Rauch, Michael; Parkin, Gerard
- Dalton Transactions, Vol. 47, Issue 36
Figures / Tables found in this record: