Diastereo- and Enantioselective Iridium-Catalyzed Allylation of Cyclic Ketone Enolates: Synergetic Effect of Ligands and Barium Enolates
Abstract
Here, we report asymmetric allylic alkylation of barium enolates of cyclic ketones catalyzed by a metallacyclic iridium complex containing a phosphoramidite ligand derived from (R)-1-(2-naphthyl)ethylamine. The reaction products contain adjacent quaternary and tertiary stereocenters. This process demonstrates that unstabilized cyclic ketone enolates can undergo diastereo- and enantioselective Ir-catalyzed allylic substitution reactions with the proper choice of enolate countercation. The products of these reactions can be conveniently transformed to various useful polycarbocyclic structures.
- Authors:
-
- Department of Chemistry, University of California, Berkeley, California 94720, United States
- Publication Date:
- Research Org.:
- Univ. of California, Berkeley, CA (United States)
- Sponsoring Org.:
- USDOE Office of Science (SC)
- OSTI Identifier:
- 1163792
- Alternate Identifier(s):
- OSTI ID: 1345809
- Grant/Contract Number:
- AC02-05CH11231
- Resource Type:
- Journal Article: Published Article
- Journal Name:
- Journal of the American Chemical Society
- Additional Journal Information:
- Journal Name: Journal of the American Chemical Society Journal Volume: 136 Journal Issue: 45; Journal ID: ISSN 0002-7863
- Publisher:
- American Chemical Society
- Country of Publication:
- United States
- Language:
- English
- Subject:
- 37 INORGANIC, ORGANIC, PHYSICAL, AND ANALYTICAL CHEMISTRY
Citation Formats
Chen, Wenyong, Chen, Ming, and Hartwig, John F. Diastereo- and Enantioselective Iridium-Catalyzed Allylation of Cyclic Ketone Enolates: Synergetic Effect of Ligands and Barium Enolates. United States: N. p., 2014.
Web. doi:10.1021/ja506500u.
Chen, Wenyong, Chen, Ming, & Hartwig, John F. Diastereo- and Enantioselective Iridium-Catalyzed Allylation of Cyclic Ketone Enolates: Synergetic Effect of Ligands and Barium Enolates. United States. https://doi.org/10.1021/ja506500u
Chen, Wenyong, Chen, Ming, and Hartwig, John F. 2014.
"Diastereo- and Enantioselective Iridium-Catalyzed Allylation of Cyclic Ketone Enolates: Synergetic Effect of Ligands and Barium Enolates". United States. https://doi.org/10.1021/ja506500u.
@article{osti_1163792,
title = {Diastereo- and Enantioselective Iridium-Catalyzed Allylation of Cyclic Ketone Enolates: Synergetic Effect of Ligands and Barium Enolates},
author = {Chen, Wenyong and Chen, Ming and Hartwig, John F.},
abstractNote = {Here, we report asymmetric allylic alkylation of barium enolates of cyclic ketones catalyzed by a metallacyclic iridium complex containing a phosphoramidite ligand derived from (R)-1-(2-naphthyl)ethylamine. The reaction products contain adjacent quaternary and tertiary stereocenters. This process demonstrates that unstabilized cyclic ketone enolates can undergo diastereo- and enantioselective Ir-catalyzed allylic substitution reactions with the proper choice of enolate countercation. The products of these reactions can be conveniently transformed to various useful polycarbocyclic structures.},
doi = {10.1021/ja506500u},
url = {https://www.osti.gov/biblio/1163792},
journal = {Journal of the American Chemical Society},
issn = {0002-7863},
number = 45,
volume = 136,
place = {United States},
year = {Thu Oct 30 00:00:00 EDT 2014},
month = {Thu Oct 30 00:00:00 EDT 2014}
}
Free Publicly Available Full Text
Publisher's Version of Record at https://doi.org/10.1021/ja506500u
Other availability
Cited by: 64 works
Citation information provided by
Web of Science
Web of Science
Figures / Tables:
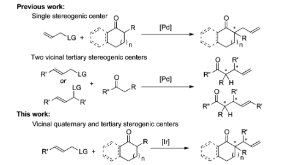 Scheme 1: Pd- and Ir-Catalyzed Allylation of Ketones
Scheme 1: Pd- and Ir-Catalyzed Allylation of Ketones
All figures and tables
(5 total)
Save to My Library
You must Sign In or Create an Account in order to save documents to your library.
Figures / Tables found in this record:
Figures/Tables have been extracted from DOE-funded journal article accepted manuscripts.