Broadband Emission in Hybrid Organic–Inorganic Halides of Group 12 Metals
- Department of Chemistry and Biochemistry, University of Oklahoma, 101 Stephenson Parkway, Norman, Oklahoma 73019, USA
- Materials Science and Technology Division, Oak Ridge National Laboratory, Oak Ridge, Tennessee 37831, USA
- Key Laboratory of Micro-Nano Measurement-Manipulation and Physics (Ministry of Education), Department of Physics, Beihang University, Beijing 100191, China
- Groupe d’Etudes de la Matière Condensée, UMR CNRS 8653-Université de Versailles Saint Quentin En Yvelines, Université Paris-Saclay, 45 Avenue des États-Unis, 78035 Versailles, France
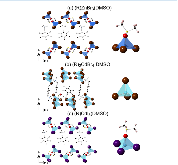
We report syntheses, crystal and electronic structures, and characterization of three new hybrid organic–inorganic halides (R)ZnBr3(DMSO), (R)2CdBr4·DMSO, and (R)CdI3(DMSO) (where (R) = C6(CH3)5CH2N(CH3)3, and DMSO = dimethyl sulfoxide). The compounds can be conveniently prepared as single crystals and bulk polycrystalline powders using a DMSO–methanol solvent system. On the basis of the single-crystal X-ray diffraction results carried out at room temperature and 100 K, all compounds have zero-dimensional (0D) crystal structures featuring alternating layers of bulky organic cations and molecular inorganic anions based on a tetrahedral coordination around group 12 metal cations. The presence of discrete molecular building blocks in the 0D structures results in localized charges and tunable room-temperature light emission, including white light for (R)ZnBr3(DMSO), bluish-white light for (R)2CdBr4·DMSO, and green for (R)CdI3(DMSO). The highest photoluminescence quantum yield (PLQY) value of 3.07% was measured for (R)ZnBr3(DMSO), which emits cold white light based on the calculated correlated color temperature (CCT) of 11,044 K. All compounds exhibit fast photoluminescence lifetimes on the timescale of tens of nanoseconds, consistent with the fast luminescence decay observed in π-conjugated organic molecules. Temperature dependence photoluminescence study showed the appearance of additional peaks around 550 nm, resulting from the organic salt emission. Density functional theory calculations show that the incorporation of both the low-gap aromatic molecule R and the relatively electropositive Zn and Cd metals can lead to exciton localization at the aromatic molecular cations, which act as luminescence centers.
- Research Organization:
- Oak Ridge National Laboratory (ORNL), Oak Ridge, TN (United States)
- Sponsoring Organization:
- USDOE Office of Science (SC), Basic Energy Sciences (BES)
- Grant/Contract Number:
- AC05-00OR22725
- OSTI ID:
- 1489084
- Alternate ID(s):
- OSTI ID: 1490568
- Journal Information:
- ACS Omega, Journal Name: ACS Omega Vol. 3 Journal Issue: 12; ISSN 2470-1343
- Publisher:
- American Chemical SocietyCopyright Statement
- Country of Publication:
- United States
- Language:
- English
Web of Science
Similar Records
Highly Efficient Broad-Band Luminescence Involving Organic and Inorganic Molecules in a Zero-Dimensional Hybrid Lead Chloride
Hybrid Organic–Inorganic Halides (C5H7N2)2MBr4 (M = Hg, Zn) with High Color Rendering Index and High-Efficiency White-Light Emission